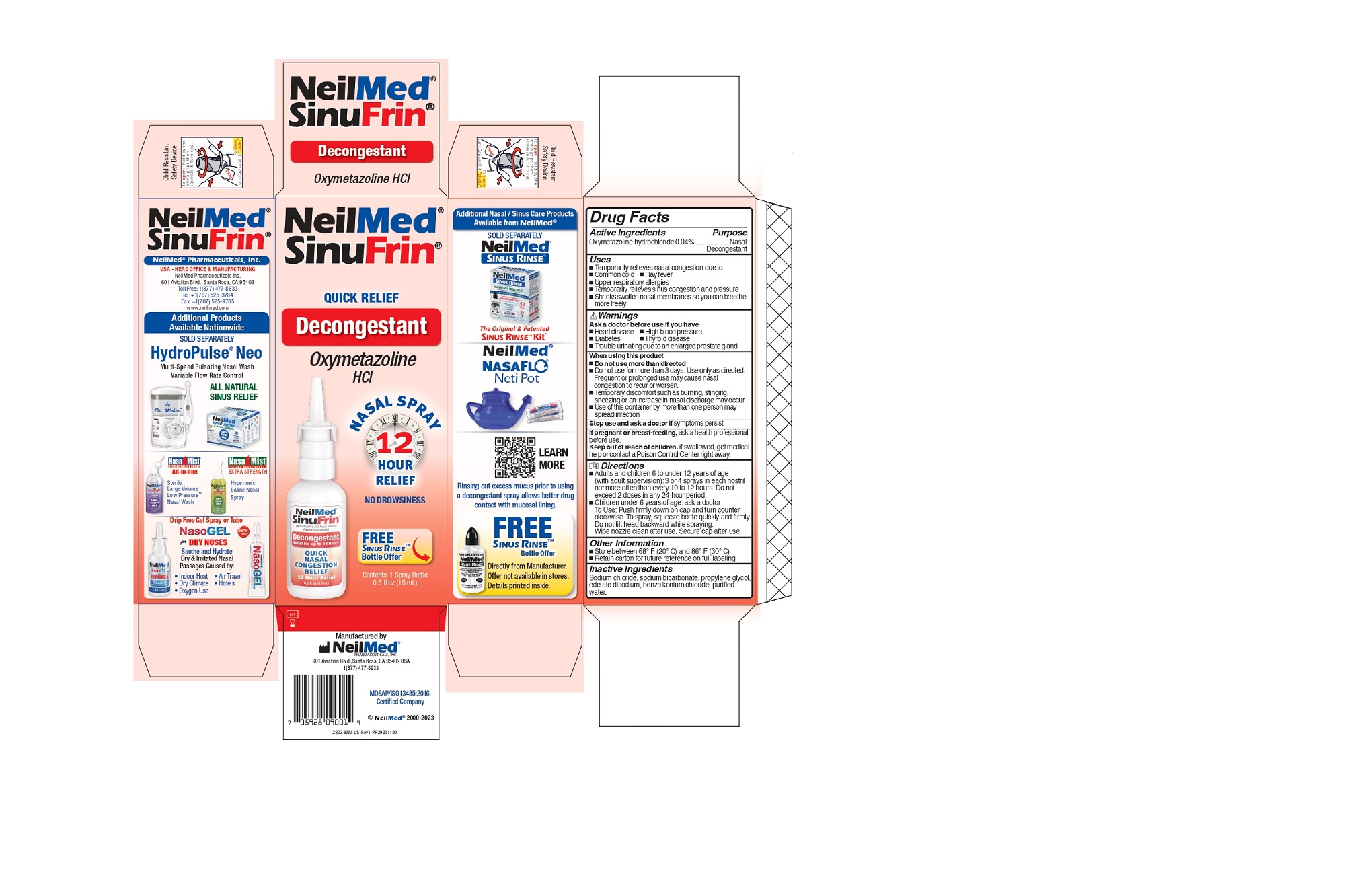
Label: SINUFRIN QUICK RELIEF DECONGESTANT- sinufrin spray
- NDC Code(s): 13709-325-01
- Packager: Neilmed pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active Ingredients
- Purpose
- Uses:
- Warnings
-
When using this product
- Do not use more than directed
- Do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- Temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- Use of this container by more than one person may spread infection.
- Warnings
- Warnings
- Warnings
-
Directions
- Adults and children 6 to under 12 years of age (with adult supervision): 3 or 4 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- Children under 6 years of age: ask a doctor To Use: Push firmly down on cap and turn counter clockwise. To spray, squeeze bottle quickly and firmly. Do not tilt head backward while spraying. Wipe nozzle clean after use. Secure cap after use.
- OTHER SAFETY INFORMATION
- Inactive Ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SINUFRIN QUICK RELIEF DECONGESTANT
sinufrin sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-325 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE (UNII: 8VLN5B44ZY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE 0.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM BICARBONATE (UNII: 8MDF5V39QO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-325-01 1 in 1 CARTON 12/11/2023 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/11/2023 Labeler - Neilmed pharmaceuticals Inc. (799295915) Establishment Name Address ID/FEI Business Operations NeilMed Pharmaceuticals Inc. 799295915 manufacture(13709-325)