Label: PONVORY- ponesimod tablet, film coated
PONVORY- ponesimod kit
-
NDC Code(s):
43068-602-00,
43068-603-00,
43068-604-00,
43068-605-00, view more43068-606-00, 43068-607-00, 43068-608-00, 43068-609-00, 43068-610-09, 43068-611-00, 43068-620-01
- Packager: Vanda Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PONVORY safely and effectively. See full prescribing information for PONVORY.
PONVORY® (ponesimod) tablets, for oral use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
INDICATIONS AND USAGE
PONVORY is a sphingosine 1-phosphate receptor modulator indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. (1)
DOSAGE AND ADMINISTRATION
- Assessments are required prior to initiating PONVORY (2.1)
- Titration is required for treatment initiation (2.2)
- The recommended maintenance dosage is 20 mg taken orally once daily (2.2)
- First-dose monitoring is recommended for patients with sinus bradycardia, first- or second-degree [Mobitz type I] atrioventricular (AV) block, or a history of myocardial infarction or heart failure (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 2 mg, 3 mg, 4 mg, 5 mg, 6 mg, 7 mg, 8 mg, 9 mg, 10 mg, and 20 mg (3)
CONTRAINDICATIONS
- In the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure (4)
- Presence of Mobitz type II second-degree, third-degree AV block, sick sinus syndrome, or sino-atrial block, unless the patient has a functioning pacemaker (4)
WARNINGS AND PRECAUTIONS
- Infections: PONVORY may increase the risk of infections. Obtain a complete blood count (CBC) before initiating treatment. Monitor for infection during treatment and for 1-2 weeks after discontinuation. Do not start PONVORY in patients with active infection. (5.1)
- Bradyarrhythmia and Atrioventricular Conduction Delays: PONVORY may result in a transient decrease in heart rate; titration is required for treatment initiation. Check an electrocardiogram (ECG) to assess for preexisting cardiac conduction abnormalities before starting PONVORY. Consider cardiology consultation for conduction abnormalities or concomitant use with other drugs that decrease heart rate. (5.2, 7.2, 7.3)
- Respiratory Effects: May cause a decline in pulmonary function. Assess pulmonary function (e.g., spirometry) if clinically indicated. (5.3)
- Liver Injury: Discontinue if significant liver injury is confirmed. Obtain liver function tests before initiating PONVORY. (5.4)
- Increased Blood Pressure (BP): Monitor BP during treatment. (5.5)
- Cutaneous Malignancies: Skin examination prior to or shortly after the start of treatment and periodically is recommended. Suspicious skin lesions should be evaluated. (5.6)
- Fetal Risk: Women of childbearing potential should use effective contraception during and for 1 week after stopping PONVORY. (5.7)
- Macular Edema: Increases the risk of macular edema. Obtain a baseline evaluation of the fundus, including the macula, near the start of treatment with PONVORY. Conduct an evaluation of the fundus, including the macula, periodically while on therapy and any time there is a change in vision. Consider discontinuing PONVORY if macular edema develops. Diabetes mellitus and uveitis increase the risk. (5.8)
ADVERSE REACTIONS
Most common adverse reactions (incidence at least 10%) are upper respiratory tract infection, hepatic transaminase elevation, and hypertension. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Vanda Pharmaceuticals, Inc. at 833-933-9331, FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Vaccines: Avoid live attenuated vaccines during and for up to 1-2 weeks after treatment with PONVORY. (7.4)
USE IN SPECIFIC POPULATIONS
Hepatic Impairment: PONVORY is not recommended in patients with moderate or severe hepatic impairment (Child-Pugh class B and C). (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of PONVORY
2.2 Recommended Dosage
2.3 First Dose Monitoring in Patients with Certain Preexisting Cardiac Conditions
2.4 Reinitiation of PONVORY After Treatment Interruption
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infections
5.2 Bradyarrhythmia and Atrioventricular Conduction Delays
5.3 Respiratory Effects
5.4 Liver Injury
5.5 Increased Blood Pressure
5.6 Cutaneous Malignancies
5.7 Fetal Risk
5.8 Macular Edema
5.9 Posterior Reversible Encephalopathy Syndrome
5.10 Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies
5.11 Severe Increase in Disability After Stopping PONVORY
5.12 Immune System Effects After Stopping PONVORY
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Anti-Neoplastic, Immune-Modulating, or Immunosuppressive Therapies
7.2 Anti-Arrhythmic Drugs, QT Prolonging Drugs, Drugs that may Decrease Heart Rate
7.3 Beta-Blockers
7.4 Vaccination
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of PONVORY
Before initiation of treatment with PONVORY, assess the following:
Cardiac Evaluation
Obtain an electrocardiogram (ECG) to determine whether preexisting conduction abnormalities are present. In patients with certain preexisting conditions, advice from a cardiologist should be sought and first-dose monitoring is recommended [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)].
Complete Blood Count
Obtain a recent (i.e., within the last 6 months or after discontinuation of prior MS therapy) complete blood count (CBC), including lymphocyte count [see Warnings and Precautions (5.1)].
Determine whether patients are taking drugs that could slow heart rate or atrioventricular (AV) conduction [see Warnings and Precautions (5.2) and Drug Interactions (7.2, 7.3)].
Liver Function Tests
Obtain recent (i.e., within the last 6 months) transaminase and bilirubin levels [see Warnings and Precautions (5.4)].
Ophthalmic Evaluation
Obtain a baseline evaluation of the fundus, including the macula, near the start of treatment with PONVORY [see Warnings and Precautions (5.8)].
Skin Examination
Obtain a baseline skin examination prior to or shortly after initiation of PONVORY. If a suspicious skin lesion is observed, it should be promptly evaluated [see Warnings and Precautions (5.6)].
Current or Prior Medications with Immune System Effects
If patients are taking anti-neoplastic, immunosuppressive, or immune-modulating therapies, or if there is a history of prior use of these drugs, consider possible unintended additive immunosuppressive effects before initiating treatment with PONVORY [see Warnings and Precautions (5.1, 5.10) and Drug Interactions (7.1)].
Vaccinations
Test patients for antibodies to varicella zoster virus (VZV) before initiating PONVORY; VZV vaccination of antibody-negative patients is recommended prior to commencing treatment with PONVORY [see Warnings and Precautions (5.1)]. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of PONVORY.
2.2 Recommended Dosage
Maintenance Dosage
After dose titration is complete (see Treatment Initiation), the recommended maintenance dosage of PONVORY is 20 mg taken orally once daily starting on Day 15.
Administer PONVORY orally once daily. Swallow the tablet whole. PONVORY can be taken with or without food.
Treatment Initiation
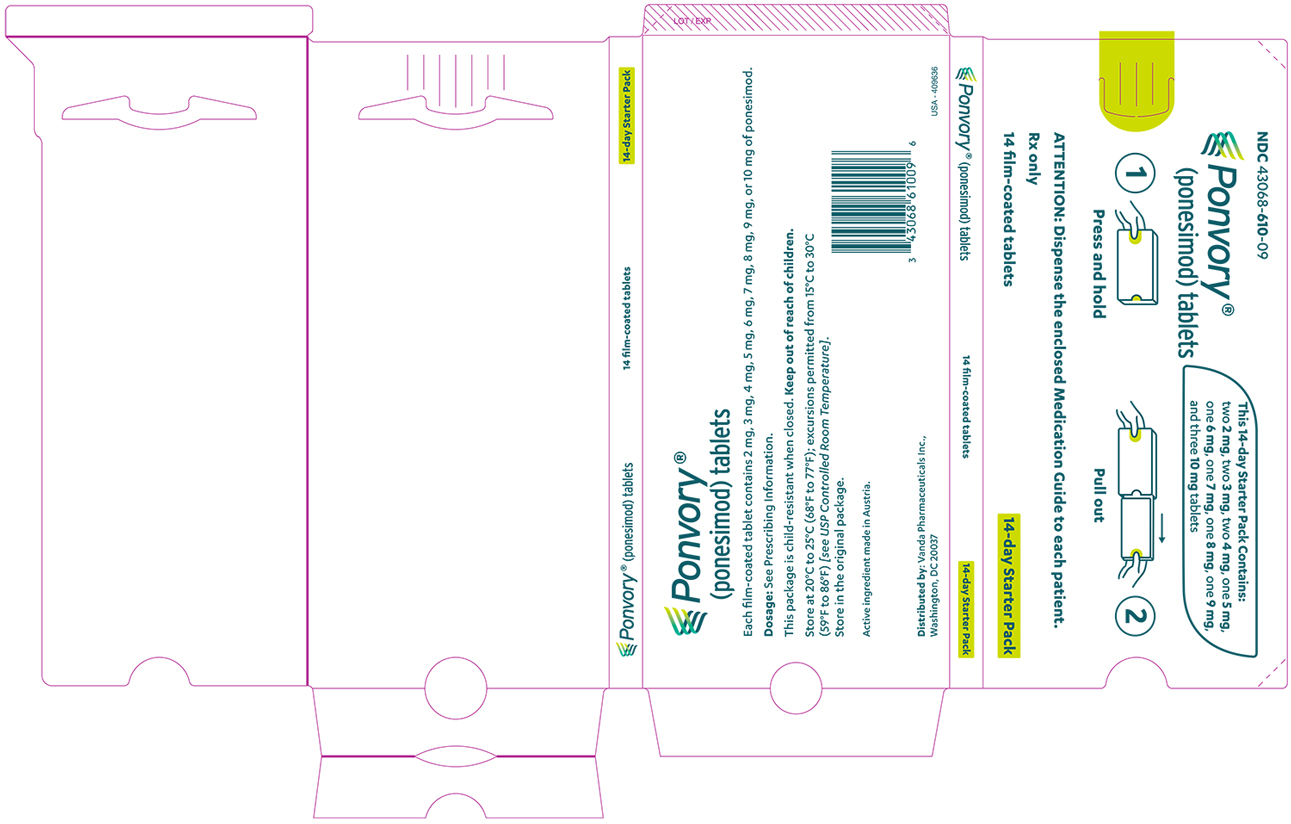
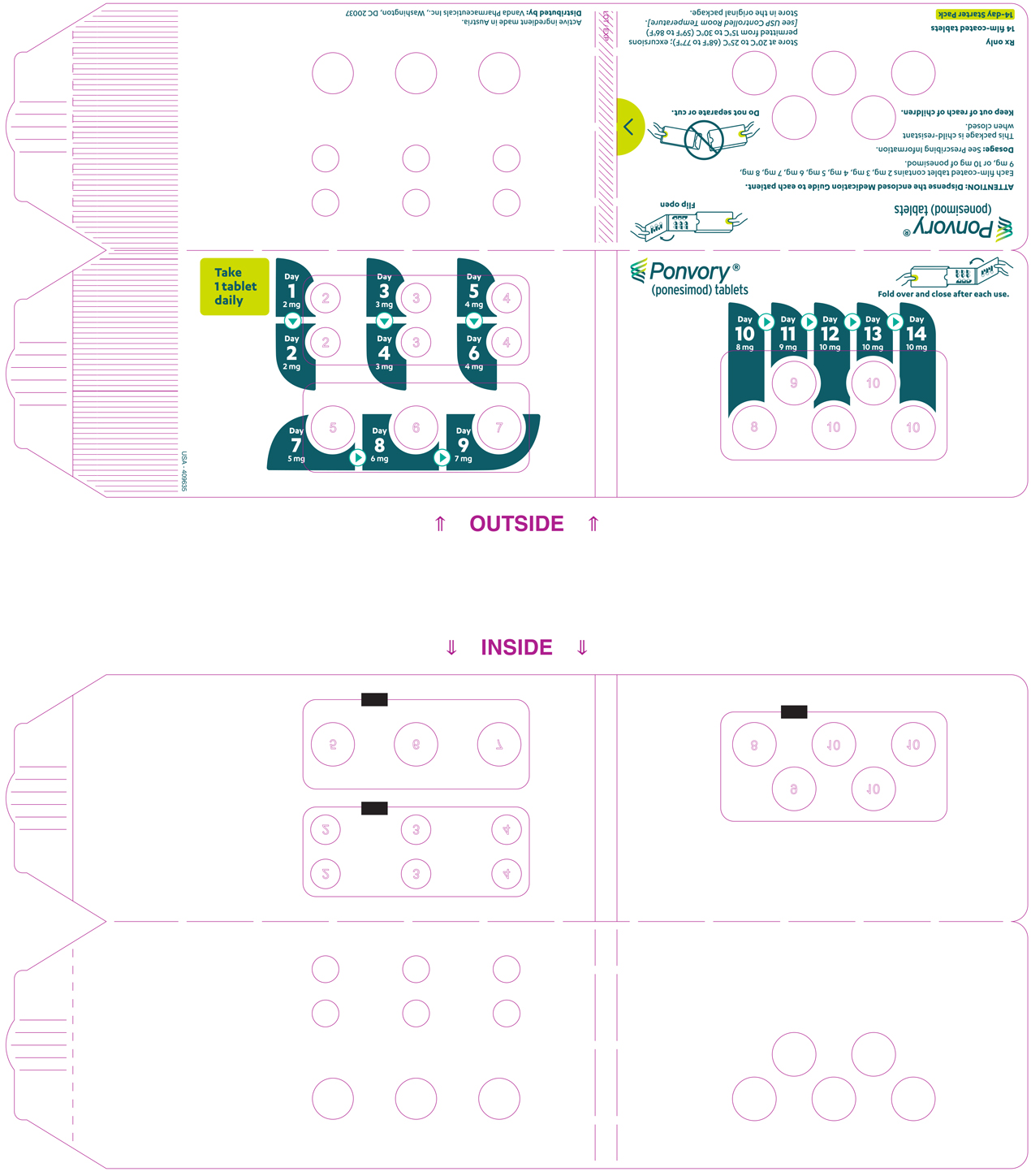
A starter pack must be used for patients initiating treatment with PONVORY [see How Supplied/Storage and Handling (16.1)]. Initiate PONVORY treatment with a 14-day titration; start with one 2 mg tablet orally once daily and progress with the titration schedule as shown in Table 1 [see Warnings and Precautions (5.2)].
Table 1: Dose Titration Regimen
Titration Day Daily Dose Days 1 and 2 2 mg Days 3 and 4 3 mg Days 5 and 6 4 mg Day 7 5 mg Day 8 6 mg Day 9 7 mg Day 10 8 mg Day 11 9 mg Days 12, 13, and 14 10 mg Maintenance Daily Dose Day 15 and thereafter 20 mg If dose titration is interrupted, missed dose instructions must be followed [see Dosage and Administration (2.4)].
2.3 First Dose Monitoring in Patients with Certain Preexisting Cardiac Conditions
Because initiation of PONVORY treatment results in a decrease in heart rate, first-dose 4-hour monitoring is recommended for patients with sinus bradycardia [heart rate less than 55 beats per minute (bpm)], first- or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure occurring more than 6 months prior to treatment initiation and in stable condition [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)].
First Dose 4-Hour Monitoring
Administer the first dose of PONVORY in a setting where resources to appropriately manage symptomatic bradycardia are available. Monitor patients for 4 hours after the first dose for signs and symptoms of bradycardia with a minimum of hourly pulse and blood pressure measurements. Obtain an ECG in these patients prior to dosing and at the end of the 4-hour observation period.
Additional Monitoring After 4-Hour Monitoring
If any of the following abnormalities are present after 4 hours (even in the absence of symptoms), continue monitoring until the abnormality resolves:
- The heart rate 4 hours post-dose is less than 45 bpm
- The heart rate 4 hours post-dose is at the lowest value post-dose, suggesting that the maximum pharmacodynamic effect on the heart may not have occurred
- The ECG 4 hours post-dose shows new onset second-degree or higher AV block
If post-dose symptomatic bradycardia, bradyarrhythmia, or conduction related symptoms occur, or if ECG 4 hours post-dose shows new onset second degree or higher AV block or QTc greater than or equal to 500 msec, initiate appropriate management, begin continuous ECG monitoring, and continue monitoring until the symptoms have resolved if no pharmacological treatment is required. If pharmacological treatment is required, continue monitoring overnight and repeat 4-hour monitoring after the second dose.
Advice from a cardiologist should be sought to determine the most appropriate monitoring strategy (which may include overnight monitoring) during treatment initiation, if treatment with PONVORY is considered in patients:
- With some preexisting heart and cerebrovascular conditions [see Warnings and Precautions (5.2)]
- With a prolonged QTc interval before dosing or during the 4-hour observation, or at additional risk for QT prolongation, or on concurrent therapy with QT prolonging drugs with a known risk of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)]
- Receiving concurrent therapy with drugs that slow heart rate or AV conduction [see Drug Interactions (7.2, 7.3)]
2.4 Reinitiation of PONVORY After Treatment Interruption
Interruption during treatment, especially during titration, is not recommended; however:
- If fewer than 4 consecutive doses are missed:
- during titration: resume treatment with the first missed titration dose and resume the titration schedule at that dose and titration day.
- during maintenance: resume treatment with the maintenance dosage.
- If 4 or more consecutive doses are missed during titration or maintenance:
- treatment should be reinitiated with Day 1 of the titration regimen (new starter pack).
If treatment needs to be reinitiated with Day 1 of the titration regimen (new starter pack), complete first-dose monitoring in patients for whom it is recommended [see Dosage and Administration (2.3)].
-
3 DOSAGE FORMS AND STRENGTHS
PONVORY is available as round, biconvex, film-coated tablets for oral use. PONVORY contains ponesimod in the following dosage strengths (see Table 2):
Table 2: Dosage Form and Strengths for PONVORY
Tablet Strength Tablet Color Tablet
SizeTablet Debossing 2 mg White 5.0 mm "2" on one side and an arch on the other side. 3 mg Red 5.0 mm "3" on one side and an arch on the other side. 4 mg Purple 5.0 mm "4" on one side and an arch on the other side. 5 mg Green 8.6 mm "5" on one side and an arch and an "A" on the other side. 6 mg White 8.6 mm "6" on one side and an arch and an "A" on the other side. 7 mg Red 8.6 mm "7" on one side and an arch and an "A" on the other side. 8 mg Purple 8.6 mm "8" on one side and an arch and an "A" on the other side. 9 mg Brown 8.6 mm "9" on one side and an arch and an "A" on the other side. 10 mg Orange 8.6 mm "10" on one side and an arch and an "A" on the other side. 20 mg Yellow 8.6 mm "20" on one side and an arch and an "A" on the other side. -
4 CONTRAINDICATIONS
PONVORY is contraindicated in patients who:
- In the last 6 months, have experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III or IV heart failure [see Warnings and Precautions (5.2)]
- Have presence of Mobitz type II second-degree, third-degree AV block, sick sinus syndrome, or sino-atrial block, unless patient has a functioning pacemaker [see Warnings and Precautions (5.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Infections
Risk of Infections
PONVORY causes a dose-dependent reduction in peripheral lymphocyte count to 30-40% of baseline values because of reversible sequestration of lymphocytes in lymphoid tissues [see Clinical Pharmacology (12.2)]. PONVORY may therefore increase the susceptibility to infections. Life-threatening and rare fatal infections have been reported in association with other sphingosine 1-phosphate (S1P) receptor modulators.
In Study 1 [see Clinical Studies (14)], the overall rate of infections was comparable between the PONVORY-treated patients and those receiving teriflunomide 14 mg (54.2% vs 52.1%, respectively). PONVORY increased the risk of upper respiratory tract infections. Serious or severe infections occurred in 1.6% of PONVORY-treated patients compared to 0.9% of patients receiving teriflunomide 14 mg.
Before initiating treatment with PONVORY, results from a recent (i.e., within 6 months or after discontinuation of prior therapy) complete blood count including lymphocyte count should be reviewed.
Initiation of treatment with PONVORY should be delayed in patients with active infection until resolution. Lymphocyte counts returned to the normal range in 90% of patients within 1 week of stopping therapy in modeling studies [see Clinical Pharmacology (12.2)]. In Study 1, peripheral lymphocyte counts returned to normal range within 2 weeks after discontinuation of PONVORY, which was the first timepoint evaluated. Because residual pharmacodynamic effects, such as lowering effects on peripheral lymphocyte count, may persist for 1 to 2 weeks after discontinuation of PONVORY, vigilance for infection should be continued for 1 to 2 weeks after PONVORY is discontinued [see Warnings and Precautions (5.12)].
In Study 1, the proportion of patients who experienced lymphocyte counts less than 0.2x109/L was 3.2%. Effective diagnostic and therapeutic strategies should be employed in patients with symptoms of infection while on therapy. Consider interruption of treatment with PONVORY if a patient develops a serious infection.
Herpes Viral Infections
Cases of herpes viral infection have been reported in the development program of PONVORY; herpes simplex encephalitis and varicella zoster meningitis have been reported with other S1P receptor modulators.
In Study 1, the rate of herpetic infections was 4.8% for both PONVORY-treated patients and those receiving teriflunomide 14 mg. Patients without a healthcare professional confirmed history of varicella (chickenpox) or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating PONVORY (see Vaccinations).
Cryptococcal Infections
Cases of fatal cryptococcal meningitis (CM) and disseminated cryptococcal infections have been reported with other S1P receptor modulators. Physicians should be vigilant for clinical symptoms or signs of CM. Patients with symptoms or signs consistent with a cryptococcal infection should undergo prompt diagnostic evaluation and treatment. PONVORY treatment should be suspended until a cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
PML has been reported in patients treated with S1P receptor modulators and other multiple sclerosis (MS) therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants, duration of use). Longer treatment duration increases the risk of PML in patients treated with S1P receptor modulators, and the majority of PML cases have occurred in patients treated with S1P receptor modulators for at least 18 months. Physicians should be vigilant for clinical symptoms or magnetic resonance imaging (MRI) findings that may be suggestive of PML. MRI findings may be apparent before clinical signs or symptoms. If PML is suspected, treatment with PONVORY should be suspended until PML has been excluded.
If PML is confirmed, treatment with PONVORY should be discontinued.
Immune reconstitution inflammatory syndrome (IRIS) has been reported in patients treated with S1P receptor modulators who developed PML and subsequently discontinued treatment. IRIS presents as a clinical decline in the patient’s condition that may be rapid, can lead to serious neurological complications or death, and is often associated with characteristic changes on MRI. The time to onset of IRIS in patients with PML was generally within a few months after S1P receptor modulator discontinuation. Monitoring for development of IRIS and appropriate treatment of the associated inflammation should be undertaken.
Prior and Concomitant Treatment with Anti-neoplastic, Immune-Modulating, or Immunosuppressive Therapies
Anti-neoplastic, immune-modulating, or immunosuppressive therapies (including corticosteroids) should be coadministered with caution because of the risk of additive immune system effects [see Drug Interactions (7.1)].
Patients without a healthcare professional confirmed history of chickenpox or without documentation of a full course of vaccination against VZV should be tested for antibodies to VZV before initiating PONVORY treatment. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with PONVORY, following which initiation of treatment with PONVORY should be postponed for 4 weeks to allow the full effect of vaccination to occur.
No clinical data are available on the efficacy and safety of vaccinations in patients taking PONVORY. Vaccinations may be less effective if administered during PONVORY treatment.
If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of PONVORY. Avoid the use of live attenuated vaccines during and for 1 to 2 weeks after treatment with PONVORY.
5.2 Bradyarrhythmia and Atrioventricular Conduction Delays
Since initiation of PONVORY treatment results in a transient decrease in heart rate and AV conduction delays, an up-titration scheme must be used to reach the maintenance dosage of PONVORY (20 mg) [see Dosage and Administration (2.2) and Clinical Pharmacology (12.2)].
Study 1 did not include patients who had:
- A resting heart rate less than 50 beats per minute (bpm) on baseline electrocardiogram
- Myocardial infarction or unstable ischemic heart disease in the last 6 months
- Cardiac failure (New York Heart Association class III-IV) or presence of any severe cardiac disease
- Cardiac conduction or rhythm disorders (including sino-atrial heart block, symptomatic bradycardia, atrial flutter or atrial fibrillation, ventricular arrhythmia, cardiac arrest) either in history or observed at screening
- Mobitz Type II second degree AV block or higher-grade AV block observed at screening
- QTcF interval greater than 470 ms (females) and greater than 450 ms (males) observed at screening
- History of syncope associated with cardiac disorders
- Uncontrolled systemic arterial hypertension
Reduction in Heart Rate
Initiation of PONVORY may result in a transient decrease in heart rate. In Study 1, bradycardia at treatment initiation and sinus bradycardia on ECG (defined as heart rate less than 50 bpm) occurred in 5.8% of PONVORY-treated patients compared to 1.6% of patients receiving teriflunomide 14 mg. After the first titration dose of PONVORY, the decrease in heart rate typically begins within an hour and reaches its nadir within 2-4 hours. The heart rate typically recovers to baseline levels 4-5 hours after administration. The mean decrease in heart rate on Day 1 of dosing was 6 bpm. With up-titration after Day 1, the post-dose decrease in heart rate is less pronounced. Bradycardia resolved in all patients in Study 1 without intervention and did not require discontinuation of PONVORY treatment. On Day 1, 3 patients treated with PONVORY had asymptomatic post-dose heart rate below or equal to 40 bpm; all 3 patients had baseline heart rates below 55 bpm.
Atrioventricular Conduction Delays
Initiation of PONVORY treatment has been associated with transient atrioventricular conduction delays that follow a similar temporal pattern as the observed decrease in heart rate during dose titration. In Study 1, the AV conduction delays manifested as first-degree AV block (prolonged PR interval on ECG), which occurred in 3.4% of PONVORY-treated patients and in 1.2% of patients receiving teriflunomide 14 mg. The conduction abnormalities typically were transient, asymptomatic, resolved within 24 hours, resolved without intervention, and did not require discontinuation of PONVORY treatment. In Study 1, second- and third-degree AV blocks were not reported in patients treated with PONVORY.
If treatment with PONVORY is considered, advice from a cardiologist should be sought for individuals:
- With significant QT prolongation (QTc greater than 500 msec)
- With atrial flutter/fibrillation or arrhythmia treated with Class Ia or Class III anti-arrhythmic drugs [see Drug Interactions (7.2)]
- With unstable ischemic heart disease, cardiac decompensated failure occurring more than 6 months prior to treatment initiation, history of cardiac arrest, cerebrovascular disease (TIA, stroke occurring more than 6 months prior to treatment initiation), or uncontrolled hypertension
- With a history of Mobitz Type II second degree AV block or higher-grade AV block, sick- sinus syndrome, or sino-atrial heart block [see Contraindications (4)]
Treatment Initiation Recommendations
- Obtain an ECG in all patients to determine whether preexisting conduction abnormalities are present.
- In all patients, a dose titration is recommended for initiation of PONVORY treatment to help reduce cardiac effects [see Dosage and Administration (2.2)].
- In patients with sinus bradycardia, first-or second-degree [Mobitz type I] AV block, or a history of myocardial infarction or heart failure with onset more than 6 months prior to initiation, first-dose monitoring is recommended [see Dosage and Administration (2.1, 2.3)].
- PONVORY is not recommended in patients with a history of cardiac arrest, cerebrovascular disease (e.g., TIA, stroke occurring more than 6 months prior to treatment initiation), uncontrolled hypertension, or severe untreated sleep apnea, since significant bradycardia may be poorly tolerated in these patients. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring strategy.
- Use of PONVORY in patients with a history of recurrent syncope or symptomatic bradycardia should be based on an overall benefit-risk assessment. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring.
- Experience with PONVORY is limited in patients receiving concurrent therapy with drugs that decrease heart rate (e.g., beta-blockers, non-dihydropyridine calcium channel blockers - diltiazem and verapamil, and other drugs that may decrease heart rate such as digoxin). Concomitant use of these drugs during PONVORY initiation may be associated with severe bradycardia and heart block. If treatment is considered, advice from a cardiologist should be sought prior to initiation of treatment in order to determine the most appropriate monitoring.
- For patients receiving a stable dose of a beta-blocker, the resting heart rate should be considered before introducing PONVORY treatment. If the resting heart rate is greater than 55 bpm under chronic beta-blocker treatment, PONVORY can be introduced. If resting heart rate is less than or equal to 55 bpm, beta-blocker treatment should be interrupted until the baseline heart rate is greater than 55 bpm. Treatment with PONVORY can then be initiated and treatment with a beta-blocker can be reinitiated after PONVORY has been up-titrated to the target maintenance dosage [see Drug Interactions (7.3)].
- For patients taking other drugs that decrease heart rate, treatment with PONVORY should generally not be initiated without consultation from a cardiologist because of the potential additive effect on heart rate [see Dosage and Administration (2.3) and Drug Interactions (7.2)].
Missed Dose During Treatment Initiation or Maintenance Treatment
If 4 or more consecutive daily doses are missed during treatment initiation or maintenance treatment, reinitiate Day 1 of the dose titration (new starter pack) and follow first-dose monitoring recommendations [see Dosage and Administration (2.4)].
5.3 Respiratory Effects
Dose-dependent reductions in forced expiratory volume over 1 second (FEV1) and reductions in diffusion lung capacity for carbon monoxide (DLCO) were observed in PONVORY-treated patients mostly occurring in the first month after treatment initiation. In Study 1, the reduction from baseline in percent predicted FEV1 at 2 years was 8.3% in PONVORY-treated patients compared to 4.4% in patients receiving teriflunomide 14 mg. In Study 1, 7 patients discontinued PONVORY because of pulmonary adverse events. There is insufficient information to determine the reversibility of the decrease in FEV1 or FVC after treatment discontinuation. PONVORY should be used with caution in patients with severe respiratory disease (i.e., pulmonary fibrosis, asthma, and chronic obstructive pulmonary disease). Spirometric evaluation of respiratory function should be performed during therapy with PONVORY if clinically indicated.
5.4 Liver Injury
Elevations of transaminases may occur in PONVORY-treated patients.
Obtain transaminase and bilirubin levels, if not recently available (i.e., within last 6 months) before initiation of PONVORY.
In Study 1, elevations of ALT to 5-fold the upper limit of normal (ULN) or greater occurred in 4.6% of patients treated with PONVORY compared to 2.5% of patients who received teriflunomide 14 mg. Elevation of ALT to 3-fold the ULN or greater occurred in 17.3% of patients treated with PONVORY and 8.3% of patients treated with teriflunomide 14 mg. The median time to an elevation of 3-fold the ULN was 3 months. The majority (89%) of patients with ALT increases 3-fold or greater the ULN continued treatment with PONVORY with values returning to less than three times the ULN within approximately 2-4 weeks.
In Study 1, the discontinuation rate because of elevations in hepatic enzymes was 2.3% of patients treated with PONVORY and 1.9% of patients who received teriflunomide 14 mg.
Patients who develop symptoms suggestive of hepatic dysfunction, such as unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, rash with eosinophilia, or jaundice and/or dark urine during treatment, should have hepatic enzymes checked. PONVORY should be discontinued if significant liver injury is confirmed.
No dosage adjustment is necessary in patients with mild hepatic impairment (Child-Pugh class A). PONVORY is not recommended in patients with moderate or severe hepatic impairment (Child- Pugh class B and C, respectively) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
5.5 Increased Blood Pressure
In Study 1, PONVORY-treated patients had an average increase of 2.9 mm Hg in systolic blood pressure and 2.8 mm Hg in diastolic blood pressure compared to 2.8 mm Hg and 3.1 mm Hg in patients receiving teriflunomide 14 mg, respectively. An increase in blood pressure with PONVORY was first detected after approximately 1 month of treatment initiation and persisted with continued treatment. Hypertensive events were reported as an adverse reaction in 10.1% of PONVORY-treated patients and in 9.0% of patients receiving teriflunomide 14 mg. One patient treated with PONVORY experienced a hypertensive crisis but had evidence of longstanding hypertensive heart disease. Blood pressure should be monitored during treatment with PONVORY and managed appropriately.
5.6 Cutaneous Malignancies
The risk of cutaneous malignancies (including basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma) is increased in patients treated with S1P receptor modulators. Use of PONVORY has been associated with an increased risk of BCC.
In Study 1, the incidence of basal cell carcinoma was 0.4% in PONVORY-treated patients compared to 0.2% in patients receiving teriflunomide 14 mg [see Adverse Reactions (6.1)]. Cases of other cutaneous malignancies, including melanoma and squamous cell carcinoma, have also been reported in patients treated with PONVORY and in patients treated with other S1P receptor modulators. Kaposi’s sarcoma and Merkel cell carcinoma have also been reported in patients treated with S1P receptor modulators in the postmarketing setting.
Skin examinations are recommended prior to or shortly after the start of treatment and periodically thereafter for all patients, particularly those with risk factors for skin cancer. Providers and patients are advised to monitor for suspicious skin lesions. If a suspicious skin lesion is observed, it should be promptly evaluated. As usual for patients with increased risk for skin cancer, exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using a sunscreen with a high protection factor. Concomitant phototherapy with UV-B radiation or PUVA photochemotherapy is not recommended in patients taking PONVORY.
5.7 Fetal Risk
Based on animal studies, PONVORY may cause fetal harm [see Use in Specific Populations (8.1, 8.3)]. Because it takes approximately 1 week to eliminate PONVORY from the body, women of childbearing potential should use effective contraception to avoid pregnancy during and for 1 week after stopping PONVORY treatment.
5.8 Macular Edema
S1P receptor modulators, including PONVORY, have been associated with an increased risk of macular edema. Obtain a baseline evaluation of the fundus, including the macula, near the start of treatment with PONVORY. Perform an examination of the fundus, including the macula, periodically while on therapy and any time there is a change in vision.
In Study 1, macular edema was reported in 1.1% of PONVORY-treated patients compared to none of the patients receiving teriflunomide 14 mg.
Continuation of PONVORY therapy in patients with macular edema has not been evaluated. Macular edema over an extended period of time (i.e., 6 months) can lead to permanent visual loss. Consider discontinuing PONVORY if macular edema develops; this decision should include an assessment of the potential benefits and risks for the individual patient. The risk of recurrence after rechallenge has not been evaluated.
Macular Edema in Patients with a History of Uveitis or Diabetes Mellitus
Patients with a history of uveitis and patients with diabetes mellitus are at increased risk of macular edema during therapy with S1P receptor modulators, including PONVORY.
5.9 Posterior Reversible Encephalopathy Syndrome
Rare cases of posterior reversible encephalopathy syndrome (PRES) have been reported in patients receiving a S1P receptor modulator. Such events have not been reported for PONVORY-treated patients in the development program. However, should a PONVORY-treated patient develop any unexpected neurological or psychiatric symptoms/signs (e.g., cognitive deficits, behavioral changes, cortical visual disturbances, or any other neurological cortical symptoms/signs), any symptom/sign suggestive of an increase of intracranial pressure, or accelerated neurological deterioration, the physician should promptly schedule a complete physical and neurological examination and should consider an MRI. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, PONVORY should be discontinued.
5.10 Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies
When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered in order to avoid unintended additive effects on the immune system while at the same time minimizing risk of disease reactivation, when initiating PONVORY.
Initiating treatment with PONVORY after treatment with alemtuzumab is not recommended.
5.11 Severe Increase in Disability After Stopping PONVORY
Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping PONVORY treatment. Patients should be observed for a severe increase in disability upon PONVORY discontinuation and appropriate treatment should be instituted, as required.
After stopping PONVORY in the setting of PML, monitor for development of immune reconstitution inflammatory syndrome (PML-IRIS) [see Warnings and Precautions (5.1)].
5.12 Immune System Effects After Stopping PONVORY
After stopping PONVORY therapy, ponesimod remains in the blood for up to 1 week. Starting other therapies during this interval will result in concomitant exposure to ponesimod. Lymphocyte counts returned to the normal range in 90% of patients within 1 week of stopping PONVORY therapy in modeling studies [see Clinical Pharmacology (12.2)]. However, residual pharmacodynamics effects, such as lowering effects on peripheral lymphocyte count, may persist for 1 to 2 weeks after the last dose. Use of immunosuppressants within this period may lead to an additive effect on the immune system, and therefore caution should be applied 1 to 2 weeks after the last dose of PONVORY [see Drug Interactions (7.1)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Infections [see Warnings and Precautions (5.1)]
- Bradyarrhythmia and Atrioventricular Conduction Delays [see Warnings and Precautions (5.2)]
- Respiratory Effects [see Warnings and Precautions (5.3)]
- Liver Injury [see Warnings and Precautions (5.4)]
- Increased Blood Pressure [see Warnings and Precautions (5.5)]
- Cutaneous Malignancies [see Warnings and Precautions (5.6)]
- Fetal Risk [see Warnings and Precautions (5.7)]
- Macular Edema [see Warnings and Precautions (5.8)]
- Posterior Reversible Encephalopathy Syndrome [see Warnings and Precautions (5.9)]
- Unintended Additive Immunosuppressive Effects From Prior Treatment With Immunosuppressive or Immune-Modulating Therapies [see Warnings and Precautions (5.10)]
- Severe Increase in Disability After Stopping PONVORY [see Warnings and Precautions (5.11)]
- Immune System Effects After Stopping PONVORY [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 1438 MS patients have received PONVORY at doses of at least 2 mg daily. These patients were included in Study 1 (2-year active-controlled versus teriflunomide 14 mg) [see Clinical Studies (14)] and in a Phase 2 (6-month placebo-controlled) study in patients with MS and the uncontrolled extension studies.
In Study 1, 82% of PONVORY-treated patients completed 2 years of study treatment, compared to 82.2% of patients receiving teriflunomide 14 mg. Adverse events led to discontinuation of treatment in 8.7% of PONVORY-treated patients, compared to 6% of patients receiving teriflunomide 14 mg. The most common adverse reactions (incidence at least 10%) in PONVORY-treated patients in Study 1 were upper respiratory tract infection, hepatic transaminase elevation, and hypertension. Table 3 lists adverse reactions that occurred in at least 2% of PONVORY-treated patients and at a higher rate than in patients receiving teriflunomide 14 mg.
Table 3: Adverse Reactions Reported in Study 1 Occurring in at Least 2% of PONVORY-Treated Patients and at a Higher Rate Than in Patients Receiving Teriflunomide 14 mg aIncludes the following terms: nasopharyngitis, upper respiratory tract infection, pharyngitis, respiratory tract infection, bronchitis, respiratory tract infection viral, viral upper respiratory tract infection, tracheitis, and laryngitis.
bIncludes the following terms: alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, and transaminases increased.
cIncludes the following terms: hypertension, hypertensive crisis, blood pressure increased, blood pressure systolic increased, and blood pressure diastolic increased.Adverse Reaction PONVORY
N=565
(%)Teriflunomide
14 mg
N=566
(%)Upper respiratory infectiona 37 34 Hepatic transaminase elevationb 23 12 Hypertensionc 10 9 Urinary tract infection 6 5 Dyspnea 5 1 Dizziness 5 3 Cough 4 2 Pain in extremity 4 3 Somnolence 3 2 Pyrexia 2 1 C-reactive protein increased 2 1 Hypercholesterolemia 2 1 Vertigo 2 1 In Study 1, the following adverse reactions occurred in less than 2% of PONVORY-treated patients, but at a rate at least 1% higher than in patients receiving teriflunomide 14 mg: viral infection, herpes zoster, hyperkalemia, lymphopenia [see Warnings and Precautions (5.1), and macular edema [see Warnings and Precautions (5.8)].
Adverse reactions in patients treated with PONVORY in an additional 6-month placebo-controlled study were generally similar to those in Study 1. The following additional adverse reactions occurred in at least 2% of PONVORY 20 mg-treated patients and at a higher rate than in patients receiving placebo (but did not meet the reporting rate criteria for inclusion in Study 1): rhinitis, fatigue, chest discomfort, peripheral edema, joint swelling, blood cholesterol increased, migraine, insomnia, depression, dyspepsia, dry mouth, bradycardia, back pain, and sinusitis.
Additionally, in uncontrolled extension trials, the adverse reaction of pneumonia was reported.
Seizures
In Study 1, cases of seizures were reported in 1.4% of PONVORY-treated patients, compared to 0.2% in patients receiving teriflunomide 14 mg. It is not known whether these events were related to the effects of MS, to PONVORY, or to a combination of both.
Respiratory Effects
In Study 1, dose-dependent reductions in FEV1 were observed in patients treated with PONVORY [see Warnings and Precautions (5.3)].
Malignancies
In Study 1, two cases of basal cell carcinoma (0.4%) were reported in PONVORY-treated patients, compared to one case of basal cell carcinoma (0.2%) in patients receiving teriflunomide 14 mg, and a case of malignant melanoma was reported in a PONVORY-treated patient. An increased risk of cutaneous malignancies has been reported in association with other S1P receptor modulators, including PONVORY [see Warnings and Precautions (5.6)].
-
7 DRUG INTERACTIONS
7.1 Anti-Neoplastic, Immune-Modulating, or Immunosuppressive Therapies
PONVORY has not been studied in combination with anti-neoplastic, immune-modulating, or immunosuppressive therapies. Caution should be used during concomitant administration because of the risk of additive immune effects during such therapy and in the weeks following administration [see Warnings and Precautions (5.1)].
When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered in order to avoid unintended additive effects on the immune system [see Warnings and Precautions (5.10)].
Because of the characteristics and duration of alemtuzumab immune suppressive effects, initiating treatment with PONVORY after alemtuzumab is not recommended.
PONVORY can generally be started immediately after discontinuation of beta interferon or glatiramer acetate.
7.2 Anti-Arrhythmic Drugs, QT Prolonging Drugs, Drugs that may Decrease Heart Rate
PONVORY has not been studied in patients taking QT prolonging drugs.
Class Ia (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) anti-arrhythmic drugs have been associated with cases of Torsades de Pointes in patients with bradycardia. If treatment with PONVORY is considered, advice from a cardiologist should be sought.
Because of the potential additive effects on heart rate, treatment with PONVORY should generally not be initiated in patients who are concurrently treated with QT prolonging drugs with known arrhythmogenic properties, heart rate lowering calcium channel blockers (e.g., verapamil, diltiazem), or other drugs that may decrease heart rate (e.g., digoxin) [see Warnings and Precautions (5.2) and Drug Interactions (7.3)]. If treatment with PONVORY is considered, advice from a cardiologist should be sought.
7.3 Beta-Blockers
Caution should be applied when PONVORY is initiated in patients receiving treatment with a beta-blocker because of the additive effects on lowering heart rate; temporary interruption of the beta-blocker treatment may be needed prior to initiation of PONVORY [see Warnings and Precautions (5.2)]. Beta-blocker treatment can be initiated in patients receiving stable doses of PONVORY.
7.4 Vaccination
During, and for up to 1 to 2 weeks after discontinuation of, treatment with PONVORY, vaccinations may be less effective. The use of live attenuated vaccines may carry the risk of infection and should therefore be avoided during PONVORY treatment and for 1 to 2 weeks after discontinuation of treatment with PONVORY [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of PONVORY in pregnant women. In animal studies, administration of ponesimod during pregnancy produced adverse effects on development, including embryo lethality and fetal malformations, in the absence of maternal toxicity. In rats and rabbits, visceral and skeletal malformations occurred at clinically relevant maternal ponesimod exposures (see Data). The receptor affected by ponesimod (sphingosine-1-phosphate receptor 1) has been demonstrated to have an important role in embryogenesis, including vascular and neural development.
In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
When ponesimod (0, 1, 10, or 40 mg/kg/day) was orally administered to pregnant rats during the period of organogenesis, increased incidences of fetal malformations primarily involving the limbs (syndactyly and ectrodactyly) and cardiovascular system (including ventricular septal defects) were observed at all but the lowest dose tested. A high incidence of embryofetal death was observed at the highest dose tested. Maternal toxicity was not observed, indicating a selective effect on the fetus. Plasma exposure (AUC) at the no-effect dose (1 mg/kg/day) for adverse effects on embryofetal development in rats was lower than that in humans at the recommended human dose (RHD) of 20 mg/day.
When ponesimod (0, 0.25, 1, or 4 mg/kg/day) was orally administered to pregnant rabbits during the period of organogenesis, an increase in post-implantation loss and fetal variations (visceral and skeletal) were noted at the highest dose tested. No maternal toxicity was observed. Plasma exposure at the no-effect dose (1 mg/kg/day) for adverse effects on embryofetal development in rabbits was lower than that in humans at the RHD. In a dose-range finding study in pregnant rabbits, oral administration of ponesimod (0, 6, 20, or 60 mg/kg/day) during organogenesis, an increase in embryofetal death and fetal limb malformation (brachydactyly) were observed at the lowest dose tested; at the higher doses, there were no live fetuses.
When ponesimod (5, 10, or 20 mg/kg) was orally administered to female rats throughout pregnancy and lactation, the offspring exhibited decreased survival, reduced body weight gain, and reduced fertility and reproductive performance (increases in pre- and post-implantation loss) at the highest dose tested, neurobehavioral impairment (increased locomotor activity) at the mid and high doses, and delayed sexual maturation at all doses tested. A no-effect dose for adverse effects on pre- and postnatal development in rats was not identified. Plasma exposure (AUC) in dams at the lowest dose tested was less than that in humans at the RHD.
8.2 Lactation
Risk Summary
There are no data on the presence of PONVORY in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. When ponesimod was orally administered to female rats during pregnancy and lactation, ponesimod was detected in the plasma of the offspring, suggesting excretion of ponesimod in milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PONVORY and any potential adverse effects on the breastfed infant from PONVORY or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Before initiation of PONVORY treatment, women of childbearing potential should be counseled on the potential for a serious risk to the fetus and the need for effective contraception during treatment with PONVORY [see Use in Specific Populations (8.1)]. Since it takes approximately one week to eliminate ponesimod from the body after stopping treatment, the potential risk to the fetus may persist, and women should use effective contraception during this period [see Warnings and Precautions (5.7)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Juvenile Animal Toxicity Data
Oral administration of ponesimod (0, 1, 10, 30, or 100 mg/kg/day) to young rats from postnatal day 28 to 91 resulted in lung histopathology (alveolar histiocytosis/edema) and decreased immune function (T-cell dependent antibody response) at the two highest doses tested. Decreased growth (body weight gain and/or long bone length) was observed at all but the low dose, and neurobehavioral impairment (increased locomotor activity) was observed at the highest dose tested. Decreased lymphocyte count and neurobehavioral impairment persisted at the end of a 4- week recovery period.
8.5 Geriatric Use
Clinical studies of PONVORY did not include patients 65 years of age and over to determine whether they respond differently from younger subjects. Use of PONVORY in elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
No dosage adjustment is necessary in patients with mild hepatic impairment (Child-Pugh class A) [see Clinical Pharmacology (12.3)].
PONVORY is not recommended in patients with moderate or severe hepatic impairment (Child-Pugh class B and C, respectively), as the risk of adverse reactions may be greater [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Symptoms and Signs
In patients with overdosage of PONVORY, especially upon initiation/reinitiation of treatment, it is important to observe for signs and symptoms of bradycardia as well as AV conduction blocks, which may include overnight monitoring. Regular measurements of pulse rate and blood pressure are required, and ECGs should be performed [see Warnings and Precautions (5.2, 5.5) and Clinical Pharmacology (12.2)].
Treatment
There is no specific antidote to ponesimod. Neither dialysis nor plasma exchange would result in meaningful removal of ponesimod from the body. The decrease in heart rate induced by PONVORY can be reversed by atropine.
In the event of overdose, PONVORY should be discontinued, and general supportive treatment given until clinical toxicity has been diminished or resolved. It is advisable to contact a poison control center to obtain the latest recommendations for the management of an overdose.
-
11 DESCRIPTION
PONVORY (ponesimod) is a sphingosine 1-phosphate receptor modulator.
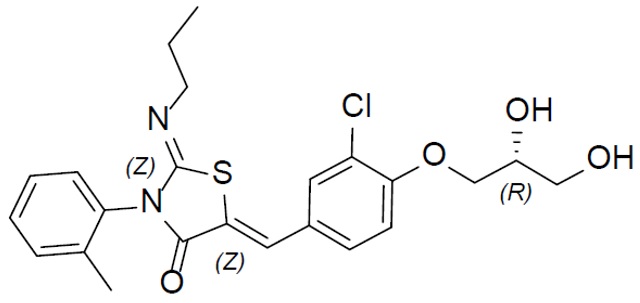
The chemical name for ponesimod is (2Z,5Z)-5-[3-chloro-4-[(2R)-2,3- dihydroxypropoxy]benzylidene]-3-(2-methylphenyl)-2-(propylimino)-1,3-thiazolidin-4-one. It has one chiral center with absolute configuration of (R). Its molecular formula is C23H25ClN2O4S and its molecular weight is 460.97 g/mol. Ponesimod has the following structural formula:
Ponesimod is a white to light yellowish powder that is practically insoluble or insoluble in water.
PONVORY® (ponesimod) tablets are provided as 2 mg, 3 mg, 4 mg, 5 mg, 6 mg, 7 mg, 8 mg, 9 mg, 10 mg, and 20 mg film-coated tablets for oral administration.
Each tablet contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone K30, silica colloidal anhydrous, and sodium lauryl sulfate.
Each tablet coating contains ferrosoferric oxide (included in 4 mg, 5 mg, 8 mg, and 9 mg film-coated tablets), hydroxypropyl methylcellulose 2910, iron oxide red (included in 3 mg, 4 mg, 7 mg, 8 mg, 9 mg, and 10 mg film-coated tablets), iron oxide yellow (included in 3 mg, 5 mg, 7 mg, 9 mg, 10 mg, and 20 mg film-coated tablets), lactose monohydrate, polyethylene glycol 3350, titanium dioxide, and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ponesimod is a S1P receptor 1 modulator that binds with high affinity to S1P receptor 1.
Ponesimod blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. The mechanism by which ponesimod exerts therapeutic effects in MS is unknown, but may involve reduction of lymphocyte migration into the central nervous system.
12.2 Pharmacodynamics
Immune System
In healthy volunteers, PONVORY induces a dose-dependent reduction of the peripheral blood lymphocyte count from a single dose of 5 mg onwards, with the greatest reduction observed 6 hours post-dose, caused by reversible sequestration of lymphocytes in lymphoid tissues. After 7 daily doses of 20 mg, the greatest decrease in absolute mean lymphocyte count was to 26% of baseline (650 cells/µL), observed 6 hours after administration. Peripheral blood B cells [CD19+] and T cells [CD3+], T-helper [CD3+CD4+], and T-cytotoxic [CD3+CD8+] cell subsets are all affected, while NK cells are not. T-helper cells were more sensitive to the effects of ponesimod than T-cytotoxic cells.
PK/PD modeling indicates lymphocyte counts returned to the normal range in greater than 90% of healthy subjects within 1 to 2 weeks of stopping therapy. In Study 1, peripheral lymphocyte counts returned to the normal range within 2 weeks after discontinuation of PONVORY.
Heart Rate and Rhythm
PONVORY causes a transient dose-dependent reduction in heart rate and AV conduction delays upon treatment initiation [see Warnings and Precautions (5.2)]. The heart rate decreases plateaued at doses greater than or equal to 40 mg [2 times the recommended maintenance dosage], and bradyarrhythmic events (AV blocks) were detected at a higher incidence under PONVORY treatment, compared to placebo. This effect starts within the first hour of dosing and is maximal at 2-4 hours post-dose. Heart rate generally returns to pre-dose values by 4-5 hours post-dose on Day 1, and the effect diminishes with repeated administration, indicating tolerance.
The decrease in heart rate induced by ponesimod can be reversed by atropine.
Beta-Blockers
The negative chronotropic effect of coadministration of PONVORY and propranolol was evaluated in a dedicated pharmacodynamics safety study. The addition of PONVORY to propranolol at steady state has an additive effect on heart rate effect [see Drug Interactions (7.3)].
Cardiac Electrophysiology
In a thorough QT study, daily administration of ponesimod doses of 40 mg and 100 mg (respectively 2- and 5-fold the recommended maintenance dose) until steady-state conditions were achieved resulted in prolongation of Fridericia-corrected QT (QTcF) intervals, with the maximum mean (upper bound of 90% two-sided confidence interval) at 11.8 ms (40 mg) and 16.2 ms (100 mg). No subject had absolute QTcF greater than 480 ms or ΔQTcF greater than 90 ms for ponesimod treatment.
Pulmonary Function
Dose-dependent reductions in FEV1 and FVC were observed in PONVORY-treated subjects, and were greater than in subjects taking placebo [see Warnings and Precautions (5.3)]. These effects can be reversed with administration of a short acting beta2 agonist.
12.3 Pharmacokinetics
Following ponesimod oral dosing, Cmax and AUC increased approximately dose-proportionally in the dose-range studied (1-75 mg). Steady-state levels are approximately 2.0- to 2.6-fold greater than with a single dose, and are achieved following 3 days of administration of the maintenance dose of ponesimod.
The pharmacokinetics of ponesimod are similar in healthy subjects and patients with MS, with 25% inter-subject variability across studies.
Absorption
The time to reach maximum plasma concentration of ponesimod is 2-4 hours post-dose. The absolute oral bioavailability of a 10 mg dose is 84%.
Food Effect
Food does not have a clinically relevant effect on ponesimod pharmacokinetics; therefore, PONVORY may be taken with or without food.
Distribution
Following IV administration in healthy subjects, the steady-state volume of distribution of ponesimod is 160 L.
Ponesimod is highly bound to plasma proteins (>99%) and is mainly (78.5%) distributed in the plasma fraction of whole blood. Animal studies show that ponesimod readily crosses the blood-brain-barrier.
Metabolism
Ponesimod is extensively metabolized prior to excretion in humans, though unchanged ponesimod was the main circulating component in plasma. Two inactive circulating metabolites, M12 and M13, have also been identified in human plasma. M13 and M12 are respectively about 20% and 6% of total drug-related exposure. Both metabolites are inactive at S1P receptors at concentrations achieved with recommended doses of ponesimod.
Experiments with human liver preparations indicate that metabolism of ponesimod to M13 occurs primarily through a combination of non-Cytochrome P450 (CYP450) enzymatic activities. Multiple CYP450 (CYP2J2, CYP3A4, CYP3A5, CYP4F3A, and CYP4F12) and non-CYP450 enzymes catalyze the oxidation of ponesimod to M12. Ponesimod also undergoes direct glucuronidation (mainly UGT1A1 and UGT2B7).
Excretion
After a single IV administration, the total clearance of ponesimod is 3.8 L/hour. The elimination half-life after oral administration is approximately 33 hours.
Following a single oral administration of 14C-ponesimod, 57% to 80% of the dose was recovered in feces (16% as unchanged ponesimod), and 10% to 18% in urine (no unchanged ponesimod).
Specific Populations
Renal Impairment
No dose adjustment is necessary in patients with renal impairment. In adult subjects with moderate or severe renal impairment (estimated creatinine clearance [CrCl], as determined by the Cockcroft-Gault, between 30-59 mL/min for moderate and <30 mL/min for severe), there were no significant changes in ponesimod Cmax and AUC, compared to subjects with normal renal function (CrCl>90 mL/min). The effect of dialysis on the PK of ponesimod has not been studied. Due to the high plasma protein binding (greater than 99%) of ponesimod, dialysis is not expected to alter the total and unbound ponesimod concentration, and no dose adjustments are anticipated based on these considerations.
Hepatic Impairment
In adult subjects with mild, moderate, or severe hepatic impairment (Child-Pugh class A, B and C, respectively), no change in ponesimod Cmax was observed, but ponesimod AUC0-∞ was increased by 1.3-, 2.0-, and 3.1-fold, respectively, compared to healthy subjects [see Use in Specific Populations (8.6)].
Age
Age (range: 17 to 65 years) was not identified to significantly influence the PK of ponesimod in population pharmacokinetics analyses. The effect of age (65 years of age and older) on the pharmacokinetics of ponesimod is unknown [see Use in Specific Populations (8.5)].
Gender
Gender has no clinically significant influence on ponesimod pharmacokinetics.
Race
No clinically relevant pharmacokinetic differences were observed between Japanese and Caucasian subjects.
Drug Interaction Studies
Beta-Blockers
In a drug-drug interaction study, the dose titration regimen of ponesimod [see Dosage and Administration (2.2)] was administered to subjects receiving propranolol (80 mg) once daily at steady state. No significant changes in pharmacokinetics of ponesimod or propranolol were observed. Compared to ponesimod alone, the combination of propranolol and the first dose of ponesimod (2 mg) led to a mean hourly heart rate decrease of 12.4 bpm (90% CI: -15.6 to -9.1). Compared to ponesimod alone, propranolol administered in combination with the first maintenance dose of ponesimod (20 mg) led to a 7.4 bpm (90% CI: -10.9 to -3.9) mean hourly heart rate decrease.
Effect of Other Drugs on Ponesimod
In vitro studies with human liver preparations indicate that metabolism of ponesimod occurs through multiple distinct enzyme systems, including multiple CYP450 (CYP2J2, CYP3A4, CYP3A5, CYP4F3A, and CYP4F12), UGT (mainly UGT1A1 and UGT2B7), and non-CYP450 oxidative enzymes, without major contribution by any single enzyme.
Ponesimod is not a substrate of P-gp, BCRP, OATP1B1, or OATP1B3 transporters. Drugs that are inhibitors of these transporters are unlikely to impact the PK of ponesimod.
Coadministration of carbamazepine 300 mg twice daily (a strong CYP3A4 and UGT1A1 inducer) at steady-state decreased ponesimod Cmax by 20% and AUC by 26%, which is not considered to be clinically significant.
Effect of Ponesimod on Other Drugs
In vitro investigations indicate that at the recommended dose of 20 mg once-daily, ponesimod and its metabolite M13 do not show any clinically relevant drug-drug interaction potential for CYP or UGT enzymes, or transporters.
Oral Contraceptives
Coadministration of ponesimod with an oral hormonal contraceptive (containing 1 mg norethisterone/norethindrone and 35 μg ethinyl estradiol) showed no clinically relevant pharmacokinetic interaction with ponesimod. Therefore, concomitant use of ponesimod is not expected to decrease the efficacy of hormonal contraceptives. No interaction studies have been performed with oral contraceptives containing other progestogens; however, an effect of ponesimod on their exposure is not expected.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Oral administration of ponesimod to mice (0, 50, 150, or 400 mg/kg/day in males and 30, 100, or 300 mg/kg/day in females) for up to 2 years resulted in incidences in hemangiosarcoma and combined hemangioma and hemangiosarcoma in males at all doses and at the highest dose tested in females. Plasma exposure (AUC) at the lowest dose tested in males (50 mg/kg/day) was approximately 5 times that in humans at the recommended human dose (RHD) of 20 mg.
Oral administration of ponesimod to rats (0, 3, 10, or 30 mg/kg/day in males and 0, 10, 30 or 100 mg/kg/day in females) for up to 2 years did not result in an increase in tumors. Plasma exposure at the highest dose tested in males (30 mg/kg/day) was approximately 4 times that in humans at the RHD of 20 mg.
Mutagenesis
Ponesimod was negative in a battery of in vitro (Ames, chromosomal aberration in mammalian cells) and in vivo (micronucleus in rat) assays.
Fertility
In separate studies, oral administration of ponesimod (0, 10, 30, or 100 mg/kg/day) to male and female rats prior to and throughout the mating period and continuing in females to Day 6 of gestation resulted in no effects on fertility. Plasma ponesimod exposures (AUC) at the highest dose tested were approximately 10 (males) and 30 (females) times that in humans at the RHD of 20 mg/day.
13.2 Animal Toxicology and/or Pharmacology
Increases in lung weight and histopathology (alveolar histiocytosis, edema) were observed in oral toxicity studies in mice, rats, and dogs. At the higher doses tested in short-term studies, alveolar histiocytosis was associated with lung edema, emphysema, or hyalinosis, and with bronchioloalveolar hyperplasia after cessation of dosing in rats and alveolar histiocytosis and hyalinosis in dogs. Effects tended to be absent or less severe after chronic treatment. These findings are considered secondary to increased vascular permeability caused by S1P1 receptor modulation. The NOAELs for lung findings in the 4-week oral toxicity studies in rats and dogs were associated with plasma exposures (AUC) similar or lower than that expected in humans at the RHD of 20 mg/day.
In dogs, coronary arterial lesions (thickening of the vessel wall, hyperplasia/hypertrophy of smooth muscles cells of the tunica media, subendocardial fibrosis) involving the papillary muscle of the left ventricle were observed in oral toxicity studies of 13 to 52 weeks in duration. At the NOAEL (2 mg/kg/day) for these findings, plasma exposures (AUC) were approximately 2 times that expected in humans at the RHD.
-
14 CLINICAL STUDIES
The efficacy of PONVORY was demonstrated in Study 1, a randomized, double-blind, parallel group, active-controlled superiority study in patients with relapsing forms of MS (NCT02425644). Patients were treated for 108 weeks. This study included patients who had an Expanded Disability Status Scale (EDSS) score of 0 to 5.5 at baseline, had experienced at least one relapse within the year prior, or two relapses within the prior 2 years, or who had at least one gadolinium-enhancing (Gd-enhancing) lesion on a brain MRI within the prior 6 months or at baseline. Patients with primary progressive MS were excluded.
Patients were randomized to receive either once daily PONVORY, beginning with a 14-day dose titration [see Dosage and Administration (2.2)] or teriflunomide 14 mg. Neurological evaluations were performed at baseline, every 3 months during the study, and at the time of a suspected relapse. Brain MRI scans were performed at baseline and at Weeks 60 and 108.
The primary endpoint was the annualized relapse rate (ARR) over the study period. Additional outcome measures included: 1) the number of new Gd-enhancing T1 lesions from baseline to Week 108, 2) the number of new or enlarging T2 lesions (without double-counting of lesions) from baseline to Week 108, and 3) the time to 3-month and 6-month confirmed disability progression. A confirmed disability progression was defined as an increase of at least 1.5 in EDSS for patients with a baseline EDSS score of 0, an increase of at least 1.0 in EDSS for patients with a baseline EDSS score of 1.0 to 5.0, or an increase of at least 0.5 in EDSS for patients with a baseline EDSS score at least 5.5, which was confirmed after 3 months and 6 months.
A total of 1133 patients were randomized to either PONVORY (N=567) or teriflunomide 14 mg (N=566); 86.4% of PONVORY-treated patients and 87.5% of teriflunomide 14 mg-treated patients completed the study as per protocol. At baseline, the mean age of patients was 37 years, 97% were White, and 65% were female. The mean disease duration was 7.6 years, the mean number of relapses in the previous year was 1.3, and the mean EDSS score was 2.6; 57% of patients had not received any prior non-steroid treatments for MS. At baseline, 42.6% of patients had one or more Gd-enhancing T1 lesions (mean 2.0) on their baseline MRI scan.
The ARR was statistically significantly lower in patients treated with PONVORY than in patients who received teriflunomide 14 mg. The number of Gd-enhancing T1 lesions and the number of new or enlarging T2 lesions were statistically significantly lower in patients treated with PONVORY than in patients who received teriflunomide 14 mg.
There was no statistically significant difference in the 3-month and 6-month confirmed disability progression outcomes between PONVORY- and teriflunomide 14 mg-treated patients over 108 weeks.
The efficacy results for Study 1 are presented in Table 4.
Table 4: Clinical and MRI Endpoints from Study 1
All analyses are based on the full analysis set (FAS), which includes all randomized patients. N refers to the number of patients included in the FAS, per treatment group.
aDefined as confirmed relapses per year through the study period (Negative binomial regression model with stratification variables (EDSS ≤ 3.5 versus EDSS > 3.5; non-steroid treatment for MS within last 2 years prior to randomization [Yes/No]) and the number of relapses in the year prior to study entry (<=1, >=2) as covariates)
bOver the study period of approximately 108 weeks
cDisability progression defined as 1.5-point increase in EDSS for patients with a baseline EDSS score of 0, 1.0-point increase in EDSS for patients with a baseline EDSS score of 1.0 to 5.0, or 0.5-point increase in EDSS for patients with a baseline EDSS score at least 5.5 confirmed 3 months later. Proportion of patients with 3-month confirmed disability progression refers to Kaplan-Meier estimates at Week 108.
dDefined as time to 3 months confirmed disability progression through the study period (Stratified Cox proportional hazard model, p-value based on the stratified log rank test)
eNot statistically significant
fCumulative number of combined unique active lesions (CUALs), defined as new or enlarging T2 lesions or Gd-enhancing T1 lesions (without double counting), mean lesions per year were 1.41 on ponesimod 20 mg (N=539), and 3.16 on teriflunomide 14 mg (N=536), a relative reduction of 56% (p<0.0001).Endpoints PONVORY 20 mg
N=567Teriflunomide 14 mg
N=566Clinical Endpoints Annualized Relapse Ratea 0.202 0.290 Relative reduction 30.5% (p=0.0003) Percentage of patients without relapseb 70.7% 60.6% Proportion of Patients with 3-month Confirmed Disability Progressionc 10.8% 13.2% Hazard Ratiod 0.83 (p=0.29)e MRI Endpointsb,f Mean number of new or enlarging T2 hyperintense lesions per year 1.40 3.16 Relative reduction 55.7% (p <.0001) Mean number of T1 Gd-enhancing lesions per MRI 0.18 0.43 Relative reduction 58.5% (p <.0001) A similar effect of PONVORY on the ARR and secondary MRI outcomes compared to teriflunomide 14 mg was observed in exploratory subgroups defined by age, gender, prior non-steroid therapy for MS, and baseline disease activity.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
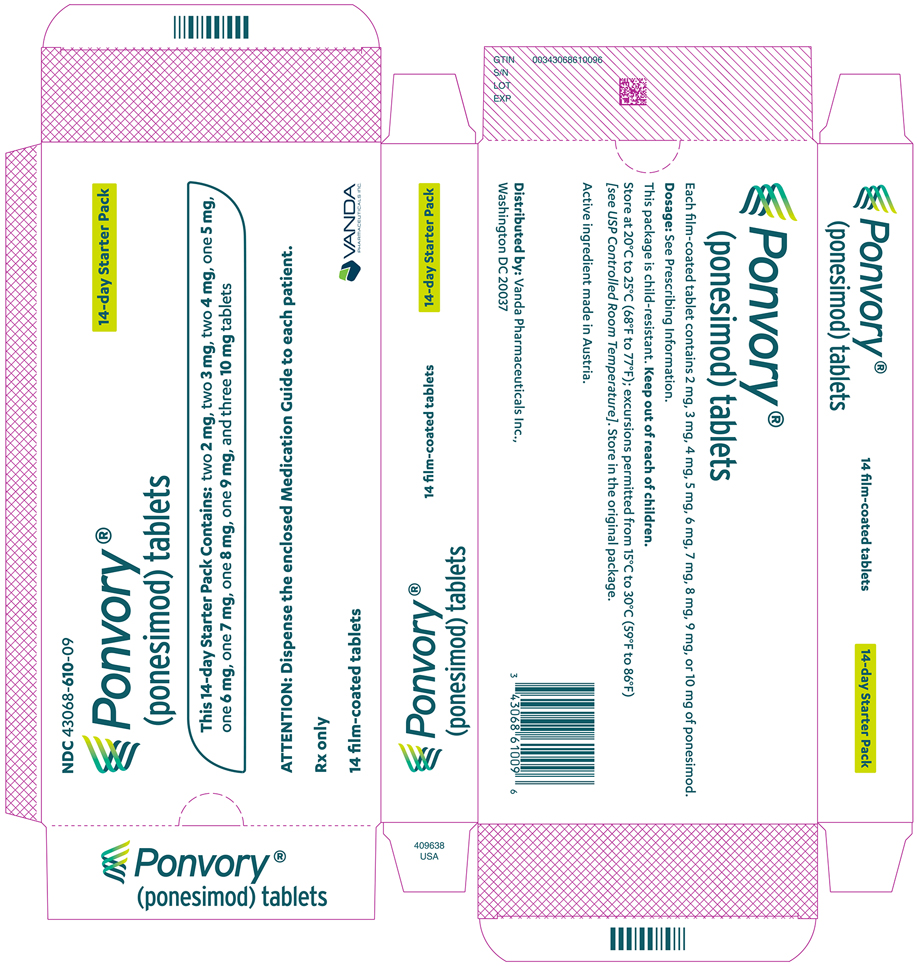
PONVORY® (ponesimod) tablet is available as round, biconvex, film-coated tablets supplied in the following dosage strengths and package configurations.
Starter Pack
Tablet Strength Tablet
ColorTablet
SizeTablet Debossing Pack Size NDC Code 2 mg White 5.0 mm "2" on one side and an arch on the other side. Child Resistant Starter
Pack
(14 tablets)NDC 43068-610-09 3 mg Red 5.0 mm "3" on one side and an arch on the other side. 4 mg Purple 5.0 mm "4" on one side and an arch on the other side. 5 mg Green 8.6 mm "5" on one side and an arch and an "A" on the other side. 6 mg White 8.6 mm "6" on one side and an arch and an "A" on the other side. 7 mg Red 8.6 mm "7" on one side and an arch and an "A" on the other side. 8 mg Purple 8.6 mm "8" on one side and an arch and an "A" on the other side. 9 mg Brown 8.6 mm "9" on one side and an arch and an "A" on the other side. 10 mg Orange 8.6 mm "10" on one side and an arch and an "A" on the other side. Maintenance Dose Bottle
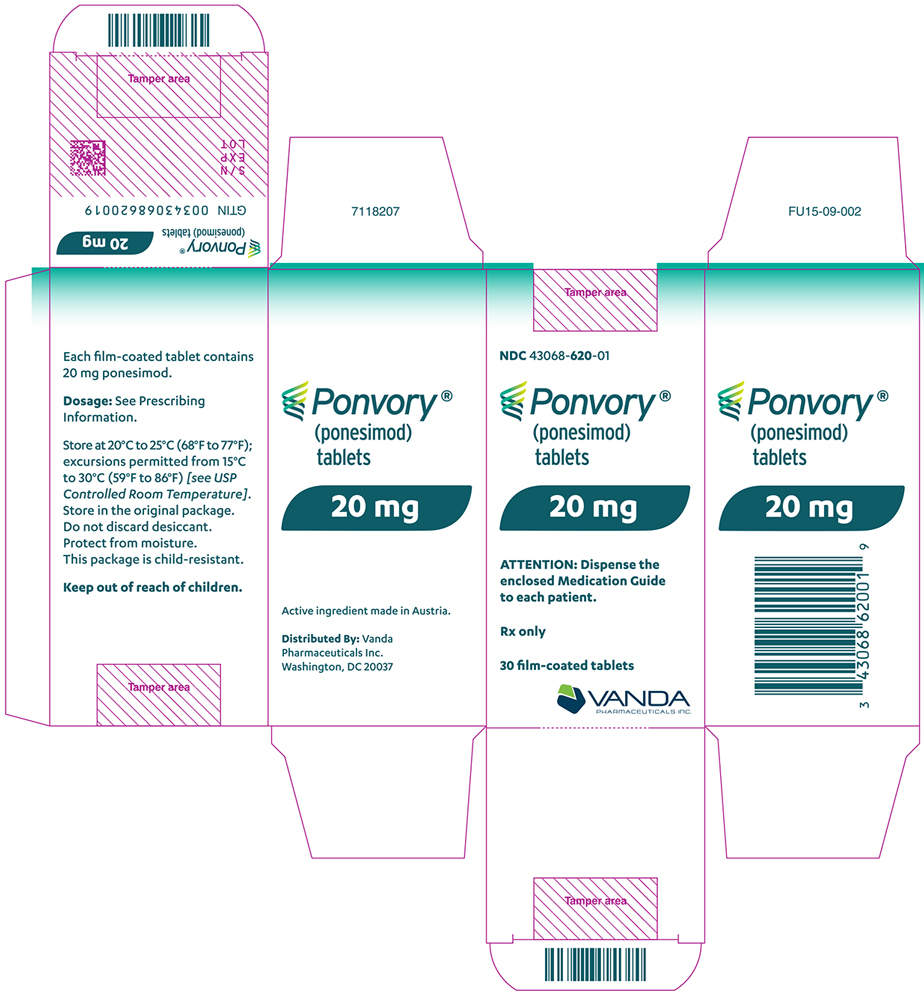
Tablet Strength Tablet
ColorTablet
SizeTablet Debossing Pack Size NDC Code 20 mg Yellow 8.6 mm "20" on one side and an arch and an "A" on the other side. Bottle of 30 tablets with child-resistant closure.
Each bottle contains a desiccant sachet.NDC 43068-620-01 16.2 Storage and Handling
Starter Pack
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see
USP Controlled Room Temperature].Store in the original package.
Maintenance Dose Bottle
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see
USP Controlled Room Temperature].Store in the original package. Do not discard desiccant. Protect from moisture.
Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Administration
Tell patients not to discontinue PONVORY without first discussing this with the prescribing healthcare provider. Advise patients to contact their healthcare provider if they accidently take more PONVORY than prescribed.
Instruct patients to administer tablets whole.
Risk of Infections
Inform patients that they may have an increased risk of infections, some of which could be life-threatening, when taking PONVORY and for 1 to 2 weeks after stopping it, and that they should contact their healthcare provider if they develop symptoms of infection [see Warnings and Precautions (5.1)]. Advise patients that the use of some vaccines containing live virus (live attenuated vaccines) should be avoided during treatment with PONVORY, and PONVORY should be paused 1 to 2 weeks prior and until 4 weeks after a planned vaccination. Recommend that patients postpone treatment with PONVORY for at least 1 month after VZV vaccination. Inform patients that prior or concomitant use of drugs that suppress the immune system may increase the risk of infection.
Inform patients that cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients treated with S1P receptor modulators. Inform the patient that PML is characterized by a progression of deficits and usually leads to death or severe disability over weeks or months. Instruct the patient of the importance of contacting their doctor if they develop any symptoms suggestive of PML. Inform the patient that typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes [see Warnings and Precautions (5.1)].
Cardiac Effects
Advise patients that initiation of PONVORY treatment results in transient decrease in heart rate [see Warnings and Precautions (5.2)]. Inform patients that to reduce this effect, dose titration is required. Advise patients that dose titration is also required if 4 or more consecutive daily doses are missed during treatment initiation or maintenance treatment [see Dosage and Administration (2.2, 2.4) and Warnings and Precautions (5.2)]. Inform certain patients with certain preexisting cardiac conditions that they will need to be observed in the doctor's office or other facility for at least 4 hours after the first dose and after reinitiation if treatment is interrupted or discontinued for certain periods [see Dosage and Administration (2.3)].
Respiratory Effects
Advise patients that they should contact their healthcare provider if they experience new onset or worsening of dyspnea [see Warnings and Precautions (5.3)].
Liver Injury
Inform patients that PONVORY may increase liver enzymes. Advise patient that they should contact their healthcare provider if they experience any unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine during treatment [see Warnings and Precautions (5.4)].
Cutaneous Malignancies
Inform patients that the risk of basal cell carcinoma is increased with the use of PONVORY and that cases of melanoma and squamous cell carcinoma have been reported. Advise patients that any suspicious skin lesions should be promptly evaluated. Advise patients to limit exposure to sunlight and ultraviolet light by wearing protective clothing and using a sunscreen with high protection factor [see Warnings and Precautions (5.6)].
Pregnancy and Fetal Risk
Inform patients that, based on animal studies, PONVORY may cause fetal harm. Discuss with women of childbearing age whether they are pregnant, might be pregnant, or are trying to become pregnant. Advise women of childbearing potential of the need for effective contraception during treatment with PONVORY and for one week after stopping PONVORY. Advise a female patient to immediately inform her healthcare provider if she is pregnant or planning to become pregnant [see Warnings and Precautions (5.7)].
Macular Edema
Advise patients that PONVORY may cause macular edema, and that they should obtain an eye exam near the start of treatment with PONVORY, have their eyes monitored periodically by an eye care professional while receiving therapy, and contact their healthcare provider if they experience any changes in their vision while taking PONVORY [see Warnings and Precautions (5.8)]. Inform patients with diabetes mellitus or a history of uveitis that their risk of macular edema is increased.
Posterior Reversible Encephalopathy Syndrome
Advise patients to immediately report to their healthcare provider any symptoms involving sudden onset of severe headache, altered mental status, visual disturbances, or seizure. Inform patients that delayed treatment could lead to permanent neurological sequelae [see Warnings and Precautions (5.9)].
Severe Increase in Disability After Stopping PONVORY
Inform patients that severe increase in disability has been reported after discontinuation of another S1P receptor modulator like PONVORY. Advise patients to contact their healthcare provider if they develop worsening symptoms of MS following discontinuation of PONVORY [see Warnings and Precautions (5.11)].
Immune System Effects After Stopping PONVORY
Advise patients that PONVORY continues to have effects, such as lowering effects on peripheral lymphocyte count, for 1 to 2 weeks after the last dose [see Warnings and Precautions (5.12)].
Active ingredient made in Austria.
Distributed by:
Vanda Pharmaceuticals, Inc.
Washington, D.C. 20037, USA© 2024 Vanda Pharmaceuticals, Inc.
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 06/2024 MEDICATION GUIDE
PONVORY ®(pon-VOR-ee)
(ponesimod)
tablets, for oral useWhat is the most important information I should know about PONVORY? PONVORY may cause serious side effects, including: -
Infections. PONVORY can increase your risk of serious infections that can be life-threatening and cause death. PONVORY lowers the number of white blood cells (lymphocytes) in your blood. This will usually go back to normal within 1 to 2 weeks of stopping treatment. Your healthcare provider should review a recent blood test of your white blood cells before you start taking PONVORY.
Call your healthcare provider right away if you have any of these symptoms of an infection during treatment with PONVORY and for 1 to 2 weeks after your last dose of PONVORY:- fever
- tiredness
- body aches
- chills
- nausea
- vomiting
- headache with fever, neck stiffness, sensitivity to light, nausea, or confusion (these may be symptoms of meningitis, an infection of the lining around your brain and spine)
Your healthcare provider may delay starting or may stop your PONVORY treatment if you have an infection.
-
Slow heart rate (bradycardia or bradyarrhythmia) when you start taking PONVORY. PONVORY can cause your heart rate to slow down, especially after you take your first dose. You should have a test to check the electrical activity of your heart called an electrocardiogram (ECG) before you take your first dose of PONVORY.
Only start your treatment with PONVORY using the Starter Pack.You must use the PONVORY Starter Pack to slowly increase the dose over a 14-day period to help reduce the effect of slowing of your heart rate. It is important to follow the recommended dosing instructions. See " How should I take PONVORY?"
Call your healthcare provider if you experience the following symptoms of slow heart rate:- dizziness
- shortness of breath
- lightheadedness
- confusion
- feeling like your heart is beating slowly or skipping beats
- chest pain
- tiredness
Follow directions from your healthcare provider when starting PONVORY and when you miss a dose. See " How should I take PONVORY?"
See "What are possible side effects of PONVORY?" for more information about side effects.
What is PONVORY? - PONVORY is a prescription medicine that is used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- It is not known if PONVORY is safe and effective in children.
Do not take PONVORY if you: - have had a heart attack, chest pain called unstable angina, stroke or mini-stroke (transient ischemic attack or TIA), or certain types of heart failure in the last 6 months.
- have certain types of heart block or irregular or abnormal heartbeat (arrhythmia), unless you have a pacemaker.
Talk to your healthcare provider before taking PONVORY if you have any of these conditions, or do not know if you have any of these conditions. Before you take PONVORY, tell your healthcare provider about all of your medical conditions, including if you: - have a fever or infection, or you are unable to fight infections due to a disease or taking medicines that weaken your immune system.
- have had chicken pox or have received the vaccine for chicken pox. Your healthcare provider may do a blood test for chicken pox virus. You may need to get the full course of vaccine for chicken pox and then wait 1 month before you start taking PONVORY.
- have slow heart rate.
- have an irregular or abnormal heartbeat (arrhythmia).
- have a history of stroke.
- have heart problems, including a heart attack or chest pain.
- have breathing problems, including during your sleep (sleep apnea).
- have liver problems.
- have high blood pressure.
- had or now have any types of skin cancer, including basal cell carcinoma (BCC), melanoma, or squamous cell carcinoma (SCC).
- have eye problems, especially an inflammation of the eye called uveitis.
- have diabetes.
- are pregnant or plan to become pregnant. PONVORY may harm your unborn baby. Talk with your healthcare provider if you are pregnant or plan to become pregnant. If you are a woman who can become pregnant, you should use effective birth control during your treatment with PONVORY and for 1 week after you stop taking PONVORY. Talk to your healthcare provider about what method of birth control is right for you during this time. Tell your healthcare provider right away if you do become pregnant while taking PONVORY or within 1 week after you stop taking PONVORY.
- are breastfeeding or plan to breastfeed. It is not known if PONVORY passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take PONVORY.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
Using PONVORY and other medicines together may affect each other causing serious side effects.Especially tell your healthcare provider if you take or have taken: - medicines to control your heart rhythm (antiarrhythmics), or blood pressure (antihypertensives), or heart beat (such as calcium channel blockers or beta-blockers).
- medicines that affect your immune system, such as alemtuzumab.
- You should not receive live vaccines during treatment with PONVORY, for at least 1 month before taking PONVORY, and for 1 to 2 weeks after you stop taking PONVORY. If you receive a live vaccine, you may get the infection the vaccine was meant to prevent. Vaccines may not work as well when given during treatment with PONVORY.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.How should I take PONVORY?
You will receive a 14-day starter pack. You must start PONVORY by slowly increasing doses over the first two weeks. Follow the dose schedule in the table below. This may reduce the risk of slowing of the heart rate.Starter Pack Day Daily Dose Day 1 2 mg tablet 1 time a day Day 2 2 mg tablet 1 time a day Day 3 3 mg tablet 1 time a day Day 4 3 mg tablet 1 time a day Day 5 4 mg tablet 1 time a day Day 6 4 mg tablet 1 time a day Day 7 5 mg tablet 1 time a day Day 8 6 mg tablet 1 time a day Day 9 7 mg tablet 1 time a day Day 10 8 mg tablet 1 time a day Day 11 9 mg tablet 1 time a day Day 12 10 mg tablet 1 time a day Day 13 10 mg tablet 1 time a day Day 14 10 mg tablet 1 time a day Maintenance Daily Dose Day 15 and thereafter 20 mg tablet 1 time a day - Take PONVORY exactly as your healthcare provider tells you to take it.
- Take PONVORY 1 time each day.
- Swallow PONVORY tablets whole.
- Take PONVORY with or without food.
- Do not stop taking PONVORY without talking with your healthcare provider first.
- Do not skip a dose.
- Start taking PONVORY with a 14-day starter pack.
- If you miss taking 1, 2, or 3 tablets in a row of PONVORY in the 14-day starter pack, continue treatment by taking the first dose you missed. Take 1 tablet as soon as you remember. Then, take 1 tablet a day to continue with the starter pack dose as planned.
- If you miss taking 1, 2, or 3 tablets in a row of PONVORY while taking the 20 mg maintenance dose, continue treatment with the 20 mg maintenance dose.
- If you miss taking 4 or more tablets in a row of PONVORY, while taking the 14-day starter pack or the 20 mg maintenance dose, you need to restart treatment with a new 14-day starter pack. Call your healthcare provider if you miss 4 or more doses of PONVORY. Do not restart PONVORY after stopping it for 4 or more days in a row without talking to your healthcare provider. If you have certain heart conditions, you may need to be monitored by your healthcare provider for at least 4 hours when you take your next dose.
- Write down the date you start taking PONVORY so you will know if you miss 4 or more doses in a row.
What are the possible side effects of PONVORY?
PONVORY may cause serious side effects, including:- See " What is the most important information I should know about PONVORY?"
- breathing problems. Some people who take PONVORY have shortness of breath. Call your healthcare provider right away if you have new or worsening breathing problems.
- liver problems. PONVORY may cause liver problems. Your healthcare provider should do blood tests to check your liver before you start taking PONVORY. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
- unexplained nausea
- vomiting
- stomach (abdominal) pain
- tiredness
- loss of appetite
- yellowing of the whites of your eyes or skin
- dark urine
- increased blood pressure. Your healthcare provider should check your blood pressure during treatment with PONVORY.
- types of skin cancer, including basal cell carcinoma, melanoma, and squamous cell carcinoma. Tell your healthcare provider if you have any changes in the appearance of your skin, including changes in a mole, a new darkened area on your skin, a sore that does not heal, or growths on your skin, such as a bump that may be shiny, pearly white, skin-colored, or pink. Your doctor should check your skin for any changes at the start of and during treatment with PONVORY. Limit the amount of time you spend in sunlight and ultraviolet (UV) light. Wear protective clothing and use a sunscreen with a high sun protection factor.
- a problem with your vision called macular edema. Macular edema can cause some of the same vision symptoms as a multiple sclerosis (MS) attack (optic neuritis). You may not notice any symptoms with macular edema. Your healthcare provider should test your vision around the time you start taking PONVORY, periodically while you continue taking PONVORY, and any time you notice vision changes during treatment with PONVORY. Your risk of macular edema is higher if you have diabetes or have had an inflammation of your eye called uveitis.
Call your healthcare provider right away if you have any of the following symptoms:
- blurriness or shadows in the center of your vision
- a blind spot in the center of your vision
- sensitivity to light
- unusually colored (tinted) vision
- swelling and narrowing of the blood vessels in your brain. A condition called Posterior Reversible Encephalopathy Syndrome (PRES) has happened with drugs in the same class. Symptoms of PRES usually get better when you stop taking PONVORY. However, if left untreated, it may lead to a stroke. Call your healthcare provider right away if you have any of the following symptoms:
- sudden severe headache
- sudden confusion
- sudden loss of vision or other changes in your vision
- seizure
- severe worsening of multiple sclerosis after stopping PONVORY. When PONVORY is stopped, symptoms of MS may return and become worse compared to before or during treatment. Always talk to your healthcare provider before you stop taking PONVORY for any reason. Tell your healthcare provider if you have worsening symptoms of MS after stopping PONVORY.
The most common side effects of PONVORY include: - upper respiratory tract infections
- elevated liver enzymes (abnormal liver tests)
- high blood pressure
For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store PONVORY? - Store PONVORY at room temperature between 68°F to 77°F (20°C to 25°C).
- Store PONVORY in the original package.
- The bottle of PONVORY contains a desiccant sachet to help keep your medicine dry (protect it from moisture). Do not throw away (discard) the desiccant.
Keep PONVORY and all medicines out of the reach of children. General information about the safe and effective use of PONVORY. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PONVORY for conditions for which it was not prescribed. Do not give PONVORY to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about PONVORY that is written for health professionals. What are the ingredients in PONVORY? Active ingredient: ponesimod Inactive ingredients: Tablet core: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone K30, silica colloidal anhydrous, and sodium lauryl sulfate. Tablet coating: ferrosoferric oxide (included in 4 mg, 5 mg, 8 mg and 9 mg film-coated tablets), hydroxypropyl methylcellulose 2910, iron oxide red (included in 3 mg, 4 mg, 7 mg, 8 mg, 9 mg and 10 mg film-coated tablets), iron oxide yellow (included in 3 mg, 5 mg, 7 mg, 9 mg, 10 mg and 20 mg film-coated tablets), lactose monohydrate, polyethylene glycol 3350, titanium dioxide, and triacetin.
Manufactured for: Vanda Pharmaceuticals, Inc., Washington, D.C. 20037, USA
© 2024 Vanda Pharmaceuticals, Inc.
For more information, go to www.ponvoryus.com or call 833-933-9331.
-
Infections. PONVORY can increase your risk of serious infections that can be life-threatening and cause death. PONVORY lowers the number of white blood cells (lymphocytes) in your blood. This will usually go back to normal within 1 to 2 weeks of stopping treatment. Your healthcare provider should review a recent blood test of your white blood cells before you start taking PONVORY.
- PRINCIPAL DISPLAY PANEL - NDC: 43068-610-09 - KIT Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 43068-610-09 - 14 Day Starter Pack Film-coated Tablets - KIT Label
- PRINCIPAL DISPLAY PANEL - NDC: 43068-610-09 - 14 Day Starter Pack Film-coated Tablets - Outside/Inside Label
- PRINCIPAL DISPLAY PANEL - NDC: 43068-620-01 - 30 Count Tablet Bottle Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 43068-620-01 - 30 Count Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
PONVORY
ponesimod tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43068-620 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 20 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSE 2910 (15000 MPA.S) (UNII: 288VBX44JC) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape ROUND Size 9mm Flavor Imprint Code 20;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-620-01 1 in 1 CARTON 11/01/2024 1 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 PONVORY
ponesimod kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43068-610 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-610-09 1 in 1 CARTON 11/01/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 2 Part 2 1 BLISTER PACK 2 Part 3 1 BLISTER PACK 2 Part 4 1 BLISTER PACK 1 Part 5 1 BLISTER PACK 1 Part 6 1 BLISTER PACK 1 Part 7 1 BLISTER PACK 1 Part 8 1 BLISTER PACK 1 Part 9 1 BLISTER PACK 3 Part 1 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-602 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 2 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 5mm Flavor Imprint Code 2;arch Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-602-00 1 in 1 CARTON 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 2 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-603 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 3 mg Product Characteristics Color RED Score no score Shape ROUND Size 5mm Flavor Imprint Code 3;arch Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-603-00 1 in 1 CARTON 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 3 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-604 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 4 mg Product Characteristics Color PURPLE Score no score Shape ROUND Size 5mm Flavor Imprint Code 4;arch Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-604-00 1 in 1 CARTON 1 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 4 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-605 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 5 mg Product Characteristics Color GREEN Score no score Shape ROUND Size 9mm Flavor Imprint Code 5;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-605-00 1 in 1 CARTON 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 5 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-606 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 6 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 9mm Flavor Imprint Code 6;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-606-00 1 in 1 CARTON 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 6 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-607 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 7 mg Product Characteristics Color RED Score no score Shape ROUND Size 9mm Flavor Imprint Code 7;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-607-00 1 in 1 CARTON 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 7 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-608 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 8 mg Product Characteristics Color PURPLE Score no score Shape ROUND Size 9mm Flavor Imprint Code 8;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-608-00 1 in 1 CARTON 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 8 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-609 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 9 mg Product Characteristics Color BROWN Score no score Shape ROUND Size 9mm Flavor Imprint Code 9;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-609-00 1 in 1 CARTON 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Part 9 of 9 PONVORY
ponesimod tablet, film coatedProduct Information Item Code (Source) NDC:43068-611 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PONESIMOD (UNII: 5G7AKV2MKP) (PONESIMOD - UNII:5G7AKV2MKP) PONESIMOD 10 mg Product Characteristics Color ORANGE Score no score Shape ROUND Size 9mm Flavor Imprint Code 10;arch;and;A Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43068-611-00 1 in 1 CARTON 1 3 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213498 03/18/2021 03/31/2027 Labeler - Vanda Pharmaceuticals Inc. (133501556)