Label: ANTI ITCH- hydrocortisone cream
- NDC Code(s): 21130-433-64
- Packager: Safeway
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
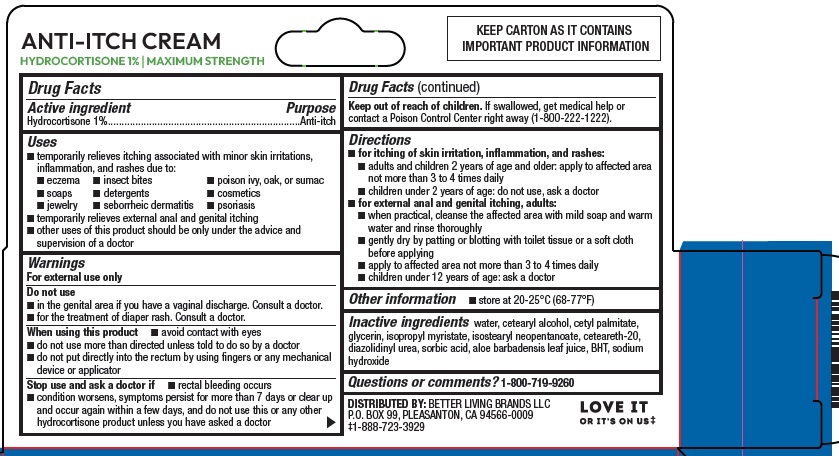
Active ingredient Hydrocortisone 1%
-
PurposeAnti-itch
-
Uses• temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to: • eczema • insect bites • poison ivy, oak, or sumac - • soaps • detergents ...
-
WarningsFor external use only - Do not use - • in the genital area if you have a vaginal discharge. Consult a doctor. • for the treatment of diaper rash. Consult a doctor. When using this ...
-
Directions• for itching of skin irritation, inflammation, and rashes: • adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily - • children under 2 years of ...
-
Other information• store at 20-25°C (68-77°F)
-
Inactive ingredientswater, cetearyl alcohol, cetyl palmitate, glycerin, isopropyl myristate, isostearyl neopentanoate, ceteareth-20, diazolidinyl urea, sorbic acid, aloe barbadensis leaf juice, BHT, sodium ...
-
Questions or comments?1-800-719-9260
-
Principal Display PanelSignature SELECT™ ANTI-ITCH CREAM - HYDROCORTISONE 1% | MAXIMUM STRENGTH - LOVE IT OR IT’S ON US - Signature SELECT™ ANTI-ITCH CREAM - HYDROCORTISONE 1% | MAXIMUM STRENGTH - NET WT 1 OZ (28 g)
-
INGREDIENTS AND APPEARANCEProduct Information