Label: CLUNIA EASYDENT- zinc gluconate liquid
- NDC Code(s): 86145-500-01
- Packager: Vetnova Animal Health LLC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
VETERINARY INDICATIONS
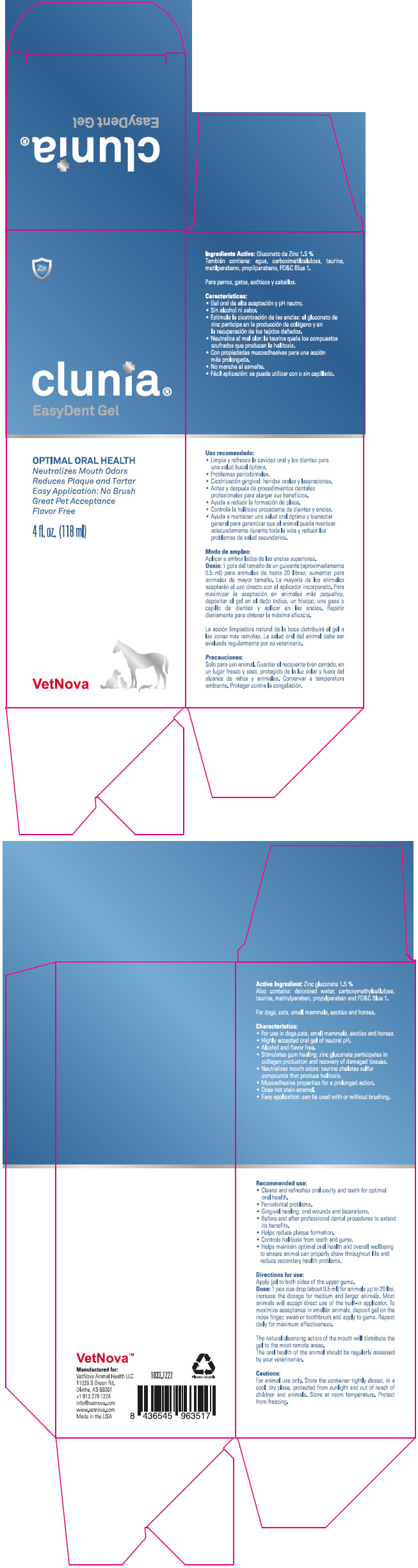
Characteristics:
- For use in dogs, cats, small mammals, exotics and horses
- Highly accepted oral gel of neutral pH.
- Alcohol and flavor free.
- Stimulates gum healing zinc gluconate participates in collagen production and recovery of damaged tissues.
- Neutralizes mouth odors: taurine chelates sulfur compounds that produce halitosis
- Mucoadhesive properties for prolonged action
- Does not stain enamel
- Easy application: can be used with or without brushing
-
INDICATIONS & USAGE
Recommended use:
- Cleans and refreshes oral cavity and teeth for optimal oral health.
- Periodontal problems.
- Gingival healing: oral wounds and lacerations.
- Before and after professional dental procedures to extend its benefits
- Helps reduce plaque formation.
- Controls halitosis from teeth and gums.
- Helps maintain optimal oral health and overall wellbeing to ensure animal can properly chew throughout life and reduce secondary health problems.
-
DOSAGE & ADMINISTRATION
Directions for use:
Apply gel to both sides of the upper gums.
Dose: 1 pea size drop (about 0.5 ml)for animals up to 20 lbs, increase the dosage for medium and larger animals. Most animals will accept direct use of the built-in applicator. To maximize acceptance in smaller animals, deposit gel on the index finger, swab or toothbrush and apply to gums. Repeat daily for maximum effectiveness.
The natural cleansing action of the mouth will distribute the gel to the most remote areas.
The oral health of the animal should be regularly assessed by your veterinarian.
- PRECAUTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118 ml Bottle Box
-
INGREDIENTS AND APPEARANCE
CLUNIA EASYDENT
zinc gluconate liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86145-500 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength zinc gluconate (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) zinc gluconate 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength Carboxymethylcellulose (UNII: 05JZI7B19X) 1 mg in 1 mL Lysine (UNII: K3Z4F929H6) taurine (UNII: 1EQV5MLY3D) Water (UNII: 059QF0KO0R) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86145-500-01 1 in 1 BOX 1 118 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 12/29/2022 Labeler - Vetnova Animal Health LLC (117666944) Registrant - BioPharmaPotentialsl LLC (053465285) Establishment Name Address ID/FEI Business Operations Vetnova Animal Health LLC 117666944 MANUFACTURE, LABEL