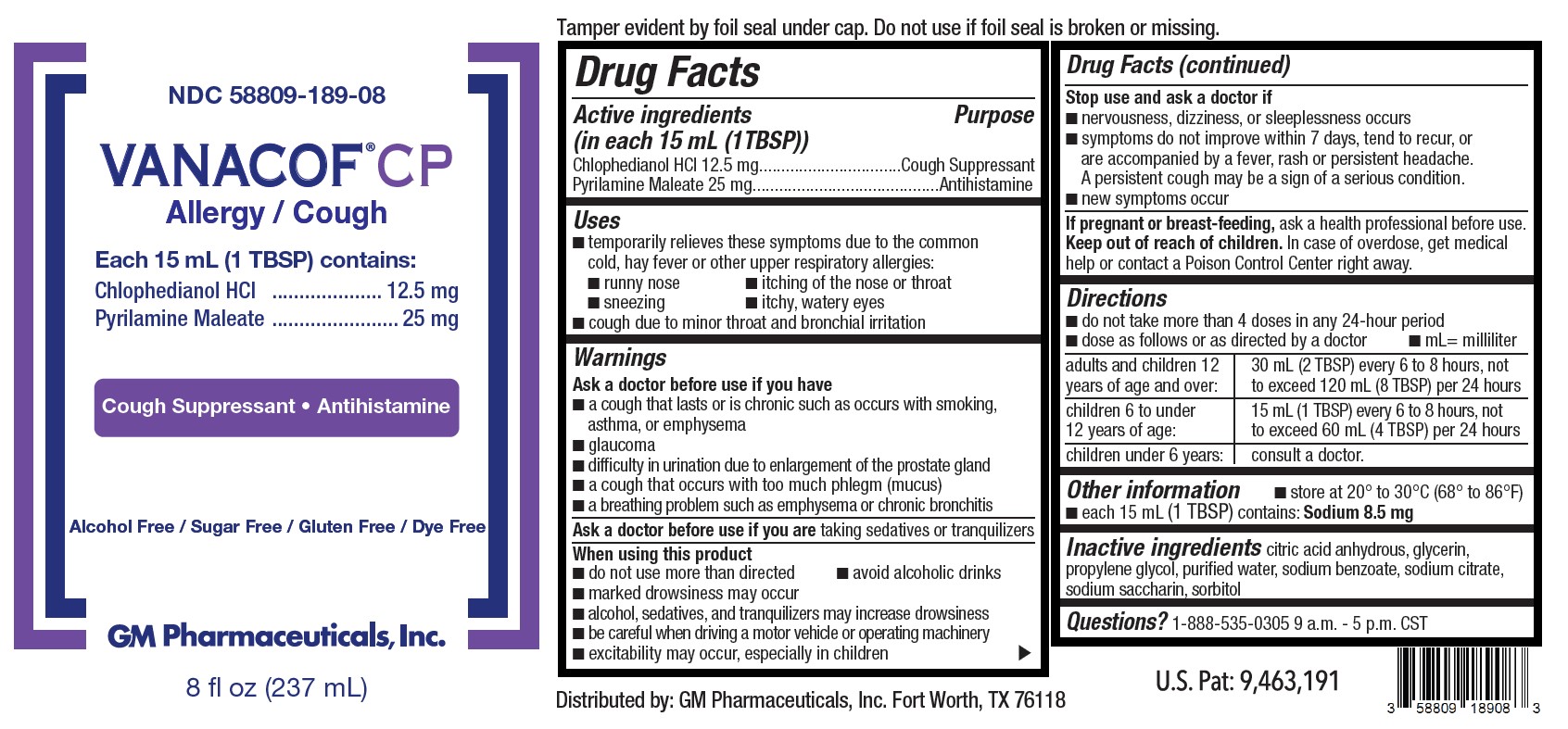
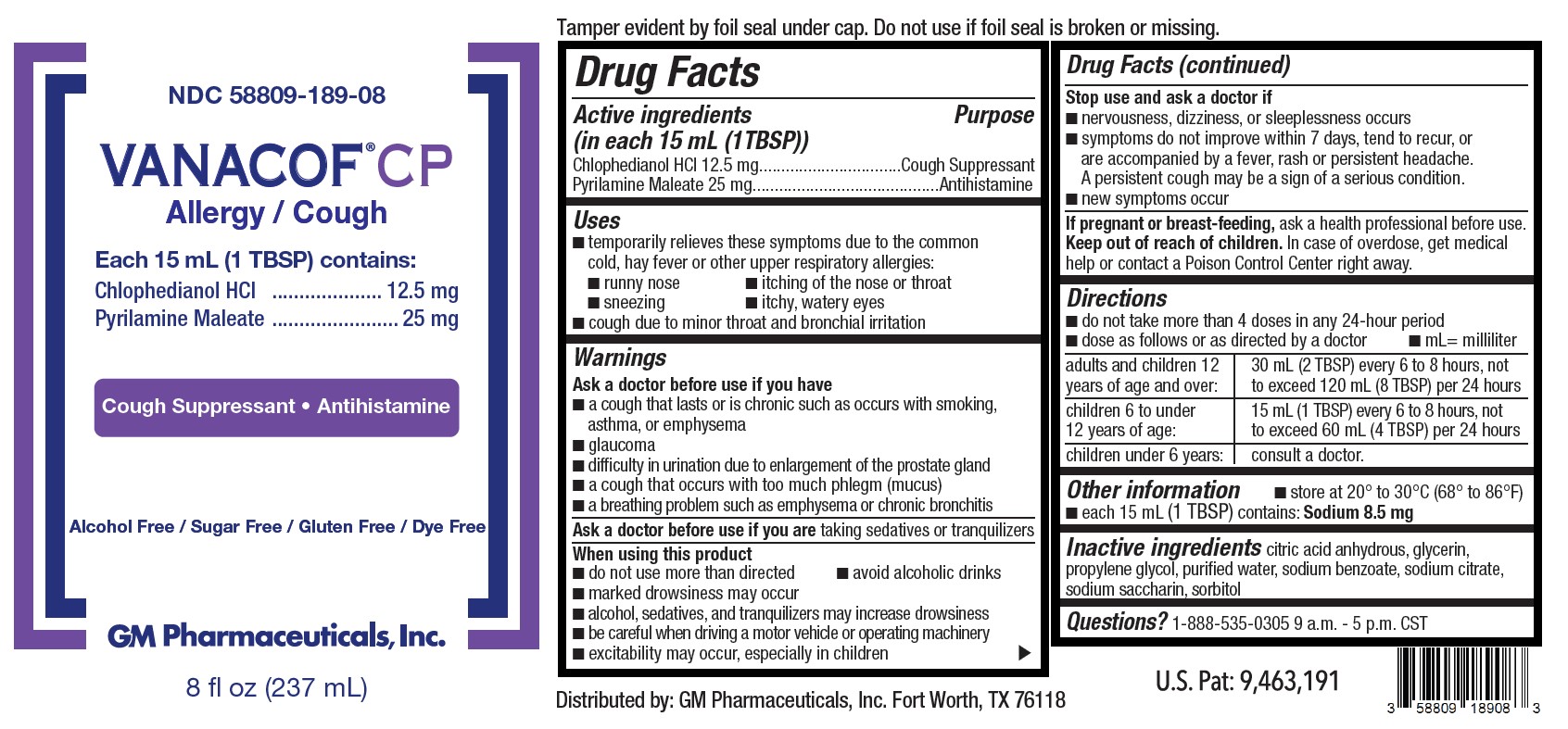
Label: VANACOF CP ALLERGY / COUGH- chlophedianol hcl, pyrilamine maleate solution
- NDC Code(s): 58809-189-08
- Packager: GM Pharmaceuticals, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in each 15 mL (1TBSP))
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
■ a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
■ glaucoma
■ difficulty in urination due to enlargement of the prostate gland
■ a cough that occurs with too much phlegm (mucus)
■ a breathing problem such as emphysema or chronic bronchitisWhen using this product
■ do not use more than directed ■ avoid alcoholic drinks
■ marked drowsiness may occur
■ alcohol, sedatives, and tranquilizers may increase drowsiness
■ be careful when driving a motor vehicle or operating machinery
■ excitability may occur, especially in children -
Directions
■ do not take more than 4 doses in any 24-hour period
■ dose as follows or as directed by a doctor ■ mL= milliliteradults and children 12 years of age and over: 30 mL (2 TBSP) every 6 to 8 hours, not to exceed 120 mL (8 TBSP) per 24 hours children 6 to under 12 years of age: 15 mL (1 TBSP) every 6 to 8 hours, not to exceed 60 mL (4 TBSP) per 24 hours children under 6 years: consult a doctor. - Other information
- Inactive ingredients
- Questions?
- OTHER SAFETY INFORMATION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VANACOF CP ALLERGY / COUGH
chlophedianol hcl, pyrilamine maleate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58809-189 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOPHEDIANOL HYDROCHLORIDE (UNII: 69QQ58998Y) (CHLOPHEDIANOL - UNII:42C50P12AP) CHLOPHEDIANOL HYDROCHLORIDE 12.5 mg in 15 mL PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 25 mg in 15 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM BENZOATE (UNII: OJ245FE5EU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58809-189-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/06/2023 Labeler - GM Pharmaceuticals, INC (793000860)