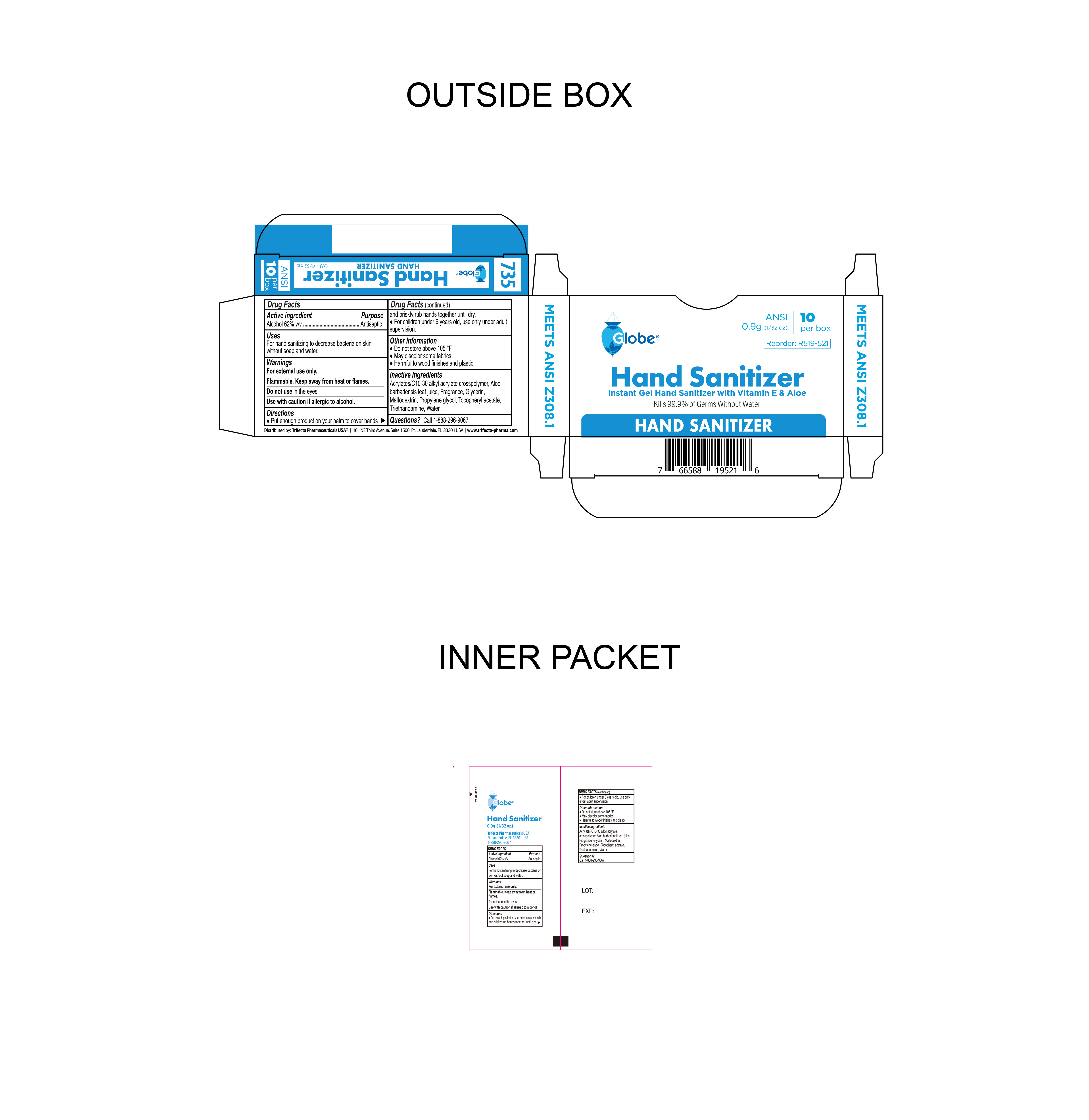
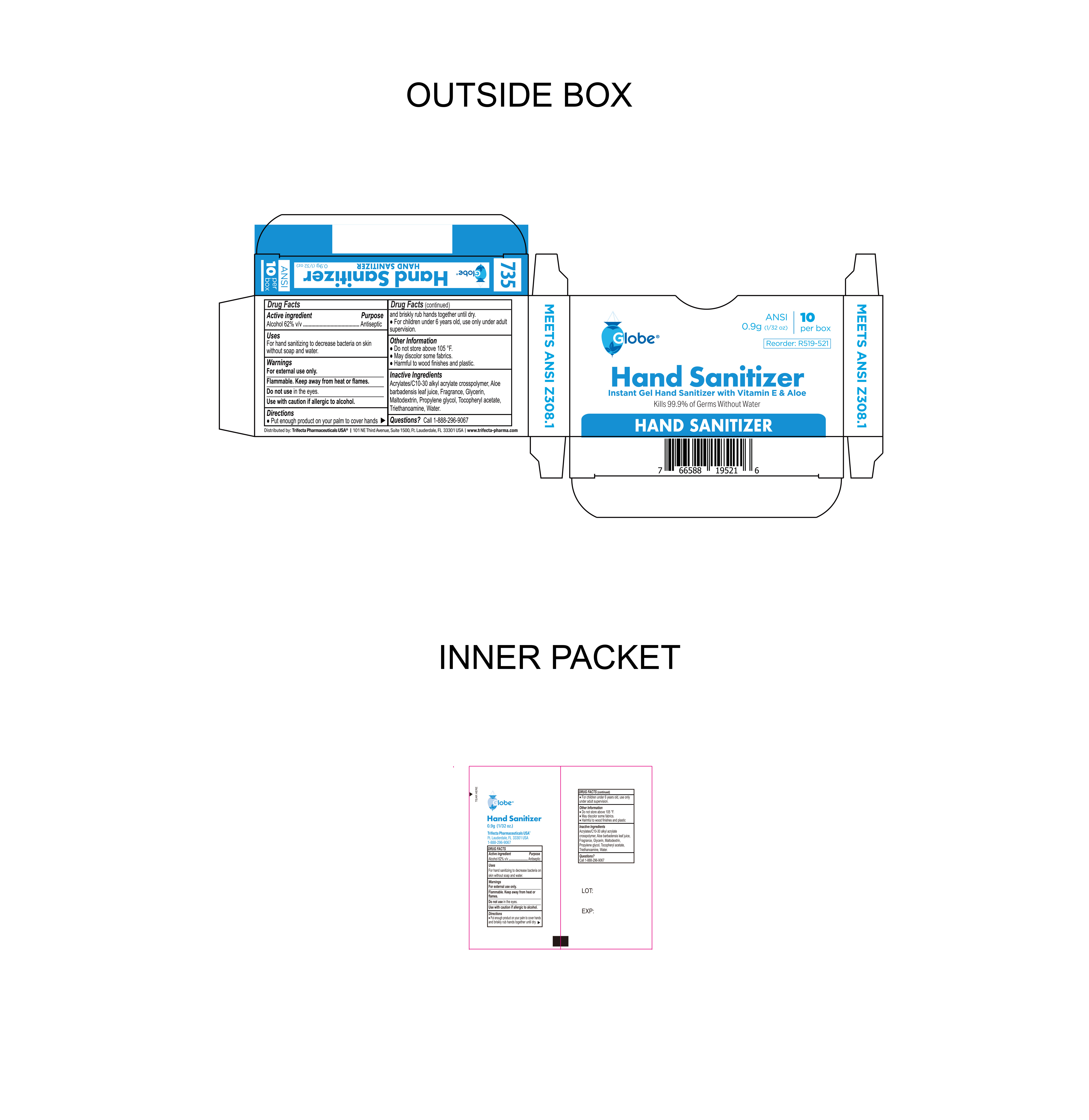
Label: HAND SANITIZER- alcohol gel
- NDC Code(s): 69396-137-10
- Packager: Trifecta Pharmaceuticals USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Directions
- Keep out of reach of Children
- Inactive Ingredients
- Other Information
- Other Information
- Package Label
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 g in 100 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) TROLAMINE (UNII: 9O3K93S3TK) FRAGRANCE 13576 (UNII: 5EM498GW35) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MALTODEXTRIN (UNII: 7CVR7L4A2D) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-137-10 10 in 1 BOX 12/03/2023 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/03/2023 Labeler - Trifecta Pharmaceuticals USA (079424163) Registrant - Trifecta Pharmaceuticals USA (079424163)