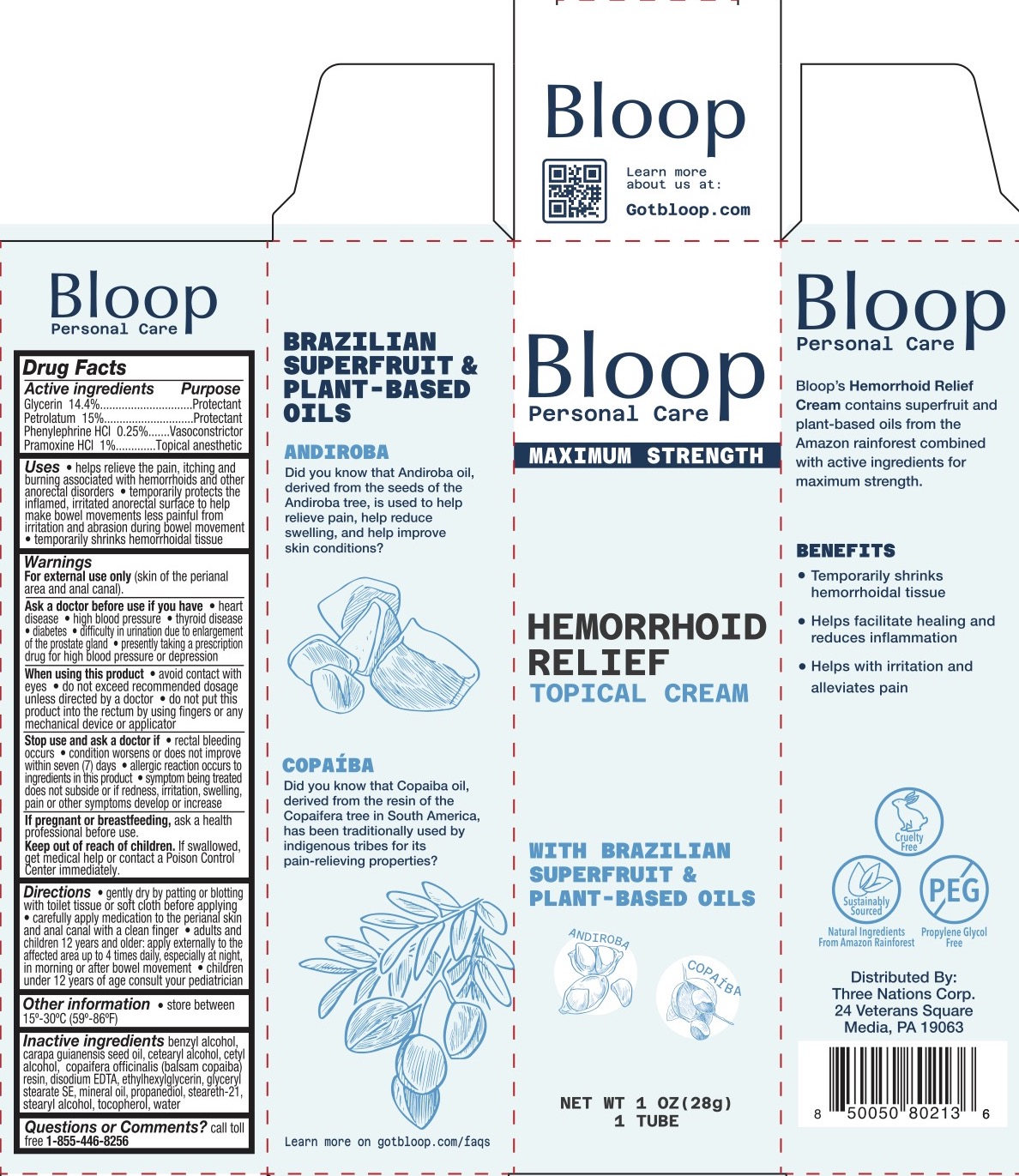
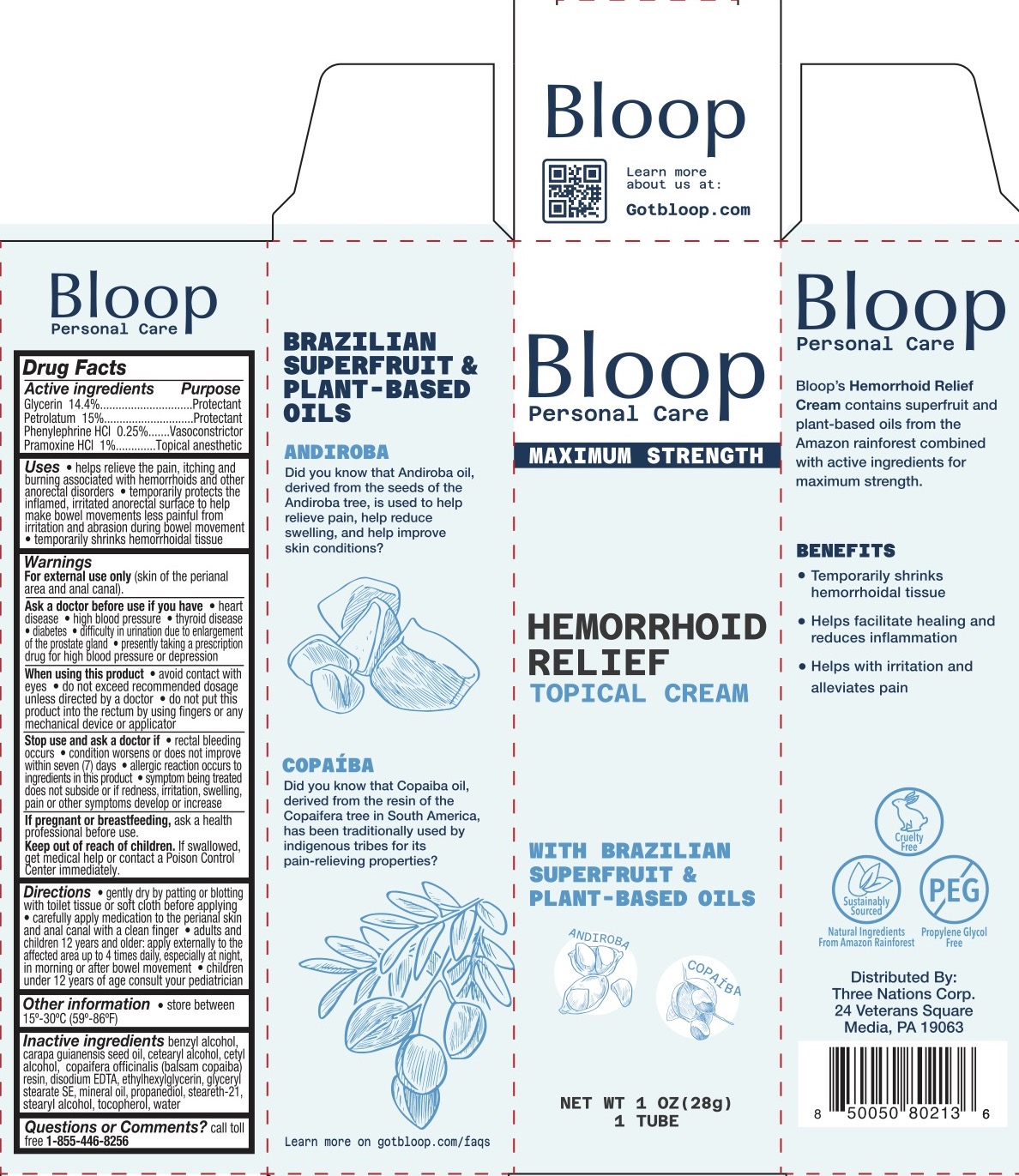
Label: BLOOP HEMORRHOID RELIEF TOPICAL- glycerin, petrolatum, phenylephrine hcl, pramoxine hcl cream
- NDC Code(s): 83838-155-01
- Packager: Three Nations Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
- Helps relieve the pain, itching, and burning associated with hemorrhoids and other anorectal disorders
- Temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful from irritation and abrasion during bowel movement.
- Temporarily shrinks hemorrhoidal tissue.
-
WARNINGS
For external use only (skin of the perianal area and anal canal).
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
- presently taking a prescription drug for high blood pressure or depression
When using this product
- avoid contact with eyes
- do not exceed recommended dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Gently dry by patting or blotting with toilet tissue or soft cloth before applying
- Carefully apply medication to the perianal skin and anal canal with a clean finger
- Adults and children 12 years and older: apply externally to the affected area up to 4 times daily, especially at night, in morning or after bowel movement
- Children under 12 years of age consult your pediatrician.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BLOOP HEMORRHOID RELIEF TOPICAL
glycerin, petrolatum, phenylephrine hcl, pramoxine hcl creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83838-155 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 14.4 g in 100 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 1 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 15 g in 100 g PHENYLEPHRINE HYDROCHLORIDE, (+/-)- (UNII: O2VT86KV7E) (PHENYLEPHRINE HYDROCHLORIDE, (+/-)- - UNII:O2VT86KV7E) PHENYLEPHRINE HYDROCHLORIDE, (+/-)- 0.25 g in 100 g Inactive Ingredients Ingredient Name Strength CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BENZYL ALCOHOL (UNII: LKG8494WBH) COPAIFERA OFFICINALIS RESIN (UNII: 1VH544O5AT) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPANEDIOL (UNII: 5965N8W85T) CETYL ALCOHOL (UNII: 936JST6JCN) STEARETH-21 (UNII: 53J3F32P58) CARAPA GUIANENSIS SEED OIL (UNII: Y82418EH2I) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83838-155-01 1 in 1 BOX 11/14/2023 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 11/14/2023 Labeler - Three Nations Corp (119050054) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(83838-155)