Label: CAROLINA CRUD CRUSHER- dextromethorphan hbr, guaifenesin syrup
- NDC Code(s): 11383-311-08
- Packager: Weeks & Leo Co., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each teaspoonful (5 mL))
- Purposes
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAROLINA CRUD CRUSHER
dextromethorphan hbr, guaifenesin syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11383-311 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 10 mL Inactive Ingredients Ingredient Name Strength HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) GLYCERIN (UNII: PDC6A3C0OX) FD&C RED NO. 40 (UNII: WZB9127XOA) WATER (UNII: 059QF0KO0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM BENZOATE (UNII: OJ245FE5EU) MENTHOL (UNII: L7T10EIP3A) Product Characteristics Color red (Red to Orange-red) Score Shape Size Flavor PINEAPPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11383-311-08 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/01/2022 Labeler - Weeks & Leo Co., Inc. (005290028) Registrant - Weeks & Leo Co., Inc. (005290028) Establishment Name Address ID/FEI Business Operations Weeks & Leo Co., Inc. 005290028 manufacture(11383-311)

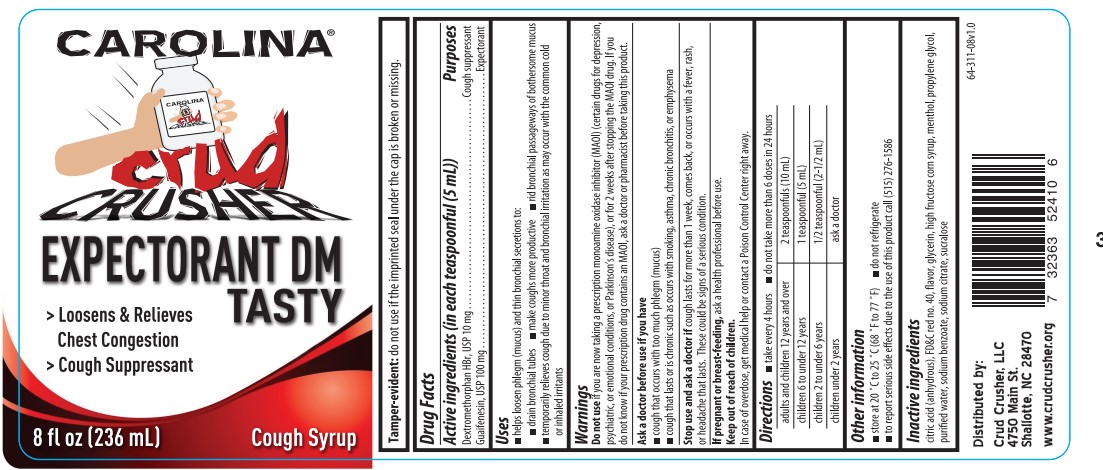
 Carolina Crud Crusher
Carolina Crud Crusher