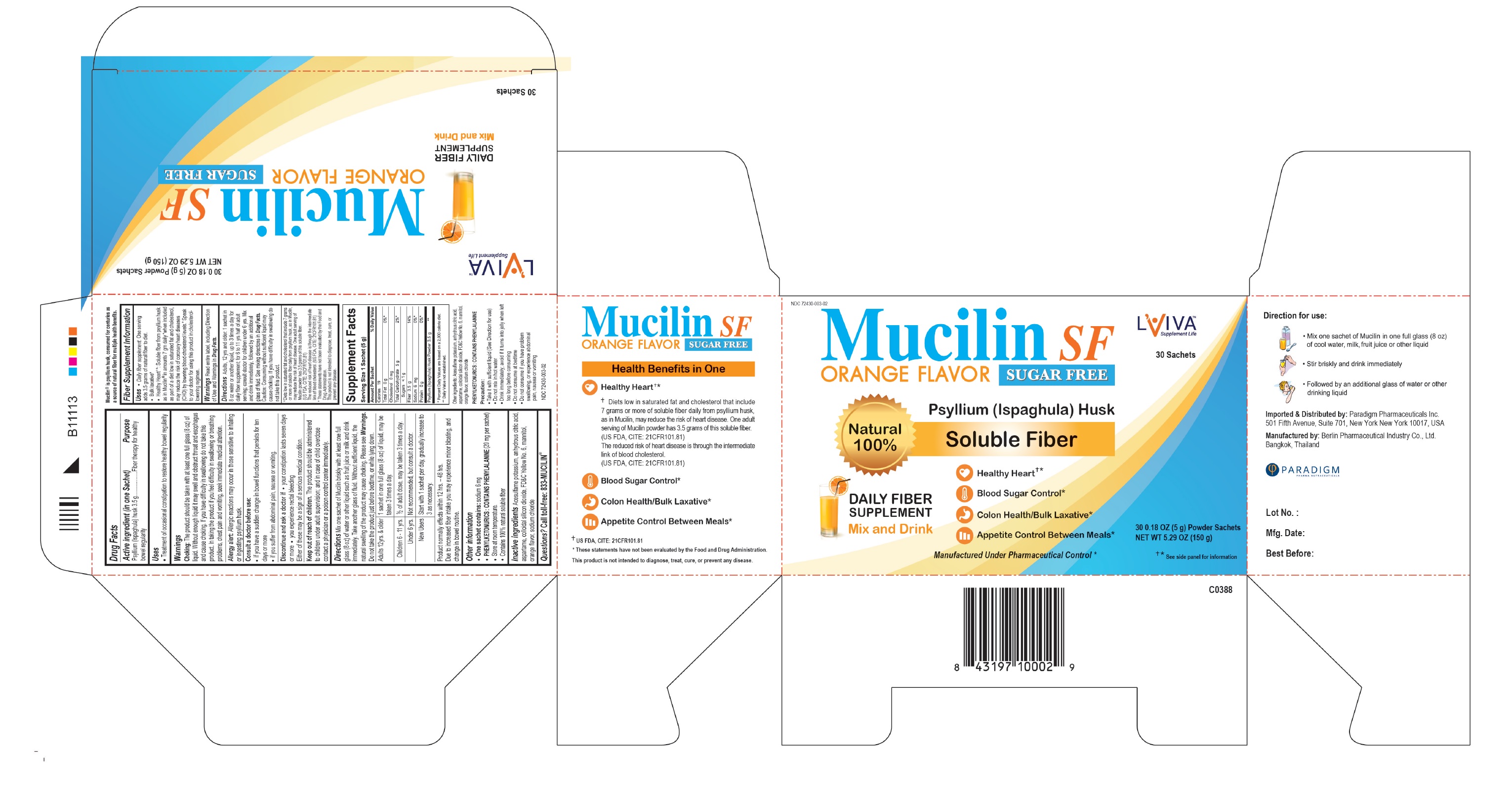
Label: MUCILIN SF (psyllium- ispaghula husk powder
- NDC Code(s): 72430-003-01, 72430-003-02
- Packager: Paradigm Pharmaceuticals Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
Choking: The product should be taken with at least one full glass (8 oz) of liquid. Without enough liquid it may swell and obstruct throat and esophagus and cause choking. If you have difficulty in swallowing do not take this product. In taking the product if you feel difficulty in swallowing, breathing problems, chest pain, vomiting, seek immediate medical attention.
Allergy alert: Allergic reactions may occur in those sensitive to inhaling or ingesting psyllium husk.
Consult a doctor before use:
- if you have a sudden change in bowel functions that persists for ten days or more
- if you suffer from abdominal pain, nausea or vomiting.
Discontinue and ask a doctor if
- your constipation lasts seven days or more
- you experience rectal bleeding
Either of these may be a sign of a serious medical condition.
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Directions for Container pack
Mix one teaspoonful of Mucilin briskly with at least one full glass (8 oz) of water or other liquid such as fruit juice or milk and drink immediately. Take another glass of fluid. Without sufficient liquid, the natural swelling of the product may cause choking. Please see Warnings. Do not take the product just before bedtime, or while lying down.
Adults 12yrs. & older
1 teaspoonful in 8 oz of liquid, may be taken 3 times a day.
Children 6 - 11 yrs.
½ of adult dose may be taken 3 times a day.
Under 6 yrs.
Not recommended, but consult a doctor.
New Users
Start with 1 dose per day, gradually increase to 3 as necessary.
Product normally effects within 12 hrs. – 48 hrs.
Due to increased fiber intake you may experience minor bloating, and change in bowel routine.
Directions for Sachet pack
Mix one sachet of Mucilin briskly with at least one full glass (8 oz) of water or other liquid such as fruit juice or milk and drink immediately. Take another glass of fluid. Without sufficient liquid, the natural swelling of the product may cause choking. Please see Warnings. Do not take the product just before bedtime, or while lying down.
Adults 12yrs. & older
1 sachet in 8 oz of liquid, may be taken 3 times a day.
Children 6 - 11 yrs.
½ of adult dose may be taken 3 times a day.
Under 6 yrs.
Not recommended, but consult a doctor.
New Users
Start with 1 dose per day, gradually increase to 3 as necessary.
Product normally effects within 12 hrs. – 48 hrs.
Due to increased fiber intake you may experience minor bloating, and change in bowel routine.
-
Other Information
Container Pack :
One teaspoonful contains: sodium 6 mg
Phenylketonurics: Contains Phenylalanine (20 mg per teaspoonful)
Store at room temperature. Tightly close the bottle to protect from humidity
Contains 100% natural soluble fiberSachet Pack
One sachet contains: sodium 6 mg
Phenylketonurics: Contains Phenylalanine (20 mg per sachet)
Store at room temperature.
Contains 100% natural soluble fiber. - Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Product Label
-
INGREDIENTS AND APPEARANCE
MUCILIN SF
psyllium (ispaghula) husk powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72430-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSYLLIUM HUSK (UNII: 0SHO53407G) (PSYLLIUM HUSK - UNII:0SHO53407G) PSYLLIUM HUSK 3.5 g in 5 g Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ASPARTAME (UNII: Z0H242BBR1) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) SODIUM CHLORIDE (UNII: 451W47IQ8X) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72430-003-02 30 in 1 BOX 01/15/2020 1 5 g in 1 POUCH; Type 0: Not a Combination Product 2 NDC:72430-003-01 400 g in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 01/15/2020 Labeler - Paradigm Pharmaceuticals Inc (080044989) Establishment Name Address ID/FEI Business Operations BERLIN PHARMACEUTICAL INDUSTRY COMPANY LIMITED - (BRANCH) 661748158 manufacture(72430-003)