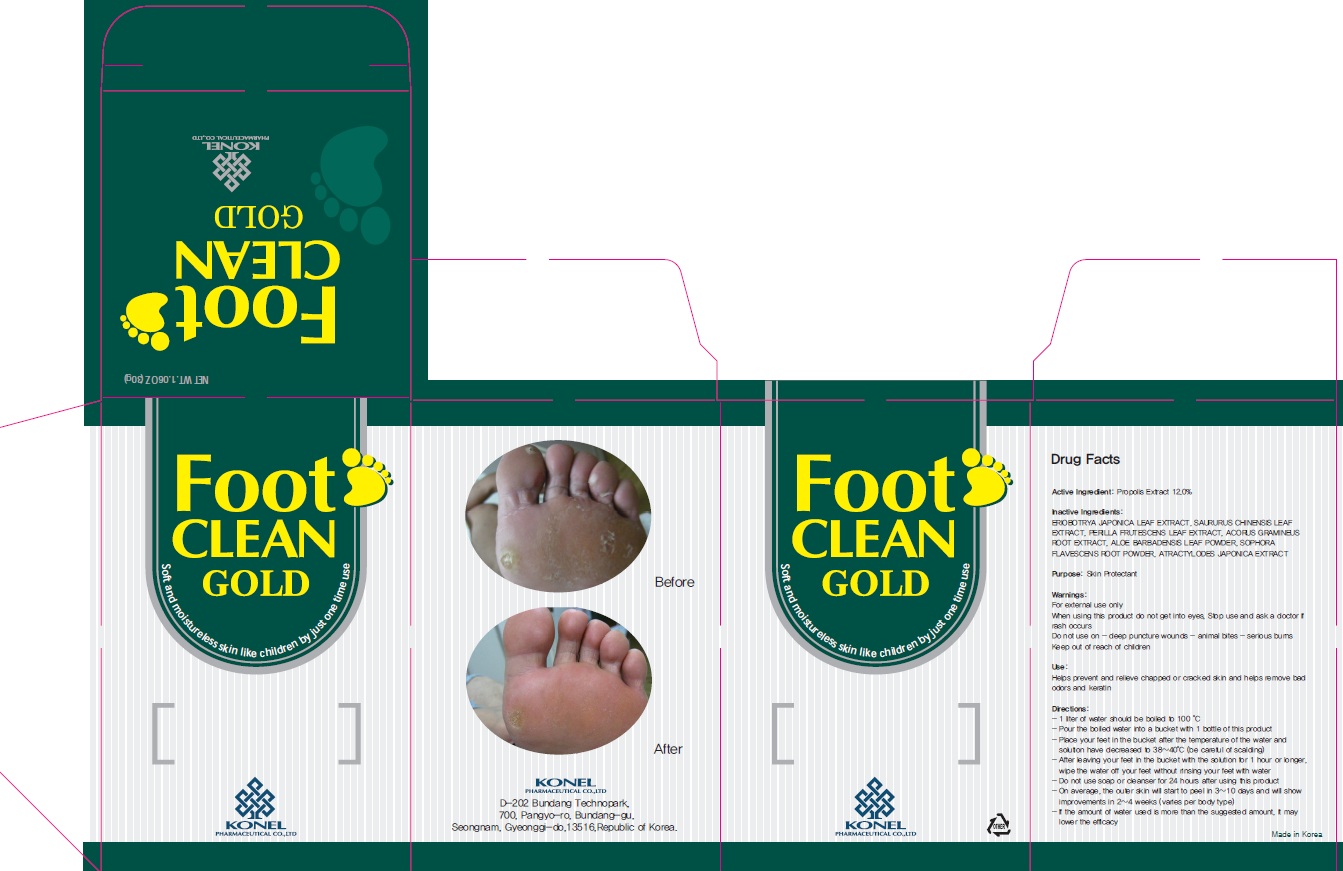
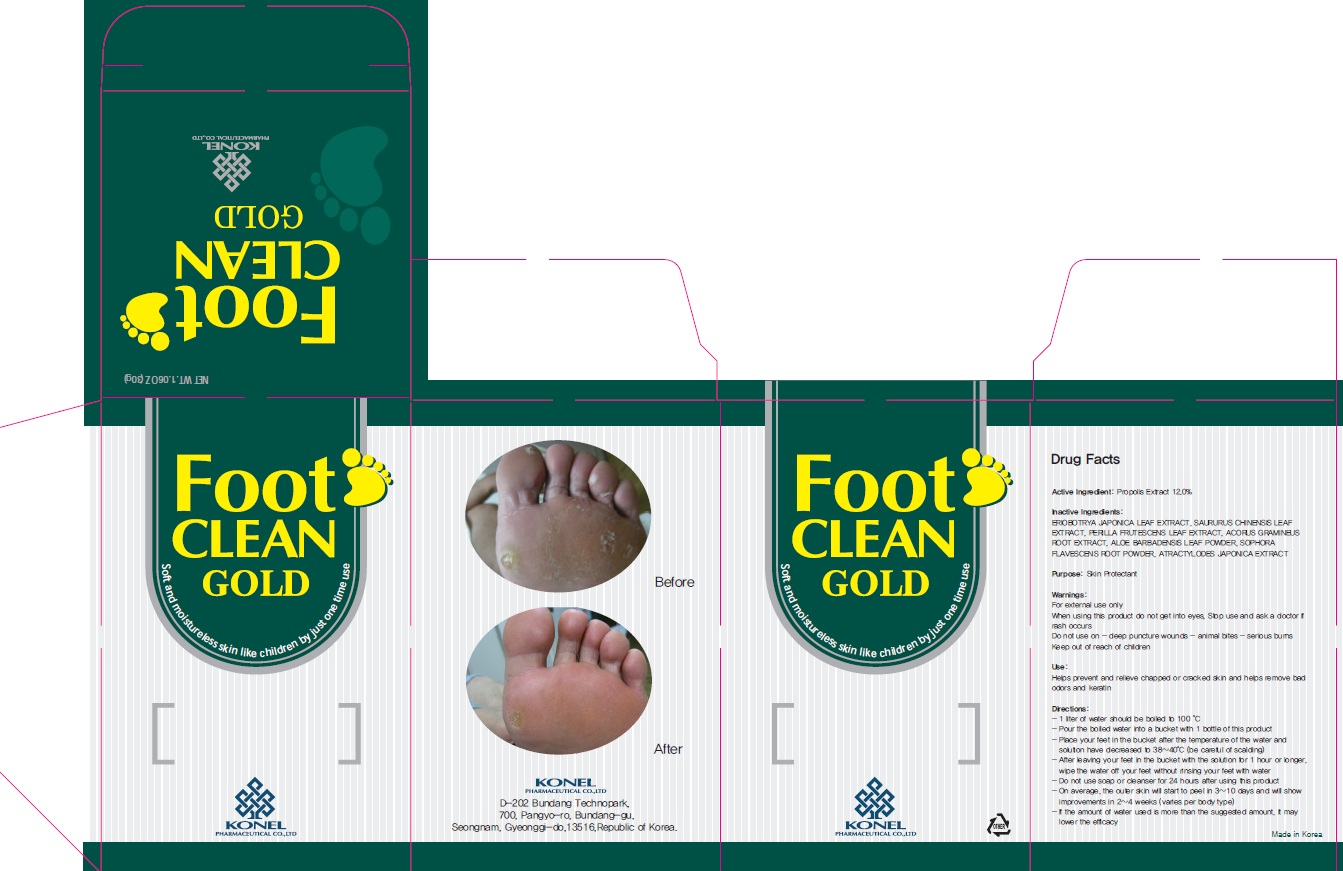
Label: FOOT CLEAN GOLD- propolis wax powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 71059-010-01, 71059-010-02 - Packager: KONEL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Use
-
Directions
Directions: - 1 liter of water should be boiled to 100 °C - Pour the boiled water into a bucket with 1 bottle of this product - Place your feet in the bucket after the temperature of the water and solution have decreased to 38~40°C (be careful of scalding) - After leaving your feet in the bucket with the solution for 1 hour or longer, wipe the water off your feet without rinsing your feet with water - Do not use soap or cleanser for 24 hours after using this product - On average, the outer skin will start to peel in 3~10 days and will show improvements in 2~4 weeks (varies per body type) - If the amount of water used is more than the suggested amount, it may lower the efficacy
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOOT CLEAN GOLD
propolis wax powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71059-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPOLIS WAX (UNII: 6Y8XYV2NOF) (PROPOLIS WAX - UNII:6Y8XYV2NOF) PROPOLIS WAX 3.6 g in 30 g Inactive Ingredients Ingredient Name Strength ERIOBOTRYA JAPONICA LEAF (UNII: Z02066SV11) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71059-010-02 1 in 1 CARTON 11/01/2016 1 NDC:71059-010-01 30 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/01/2016 Labeler - KONEL (689605036) Registrant - KONEL (689605036) Establishment Name Address ID/FEI Business Operations KONEL 689605036 manufacture(71059-010)