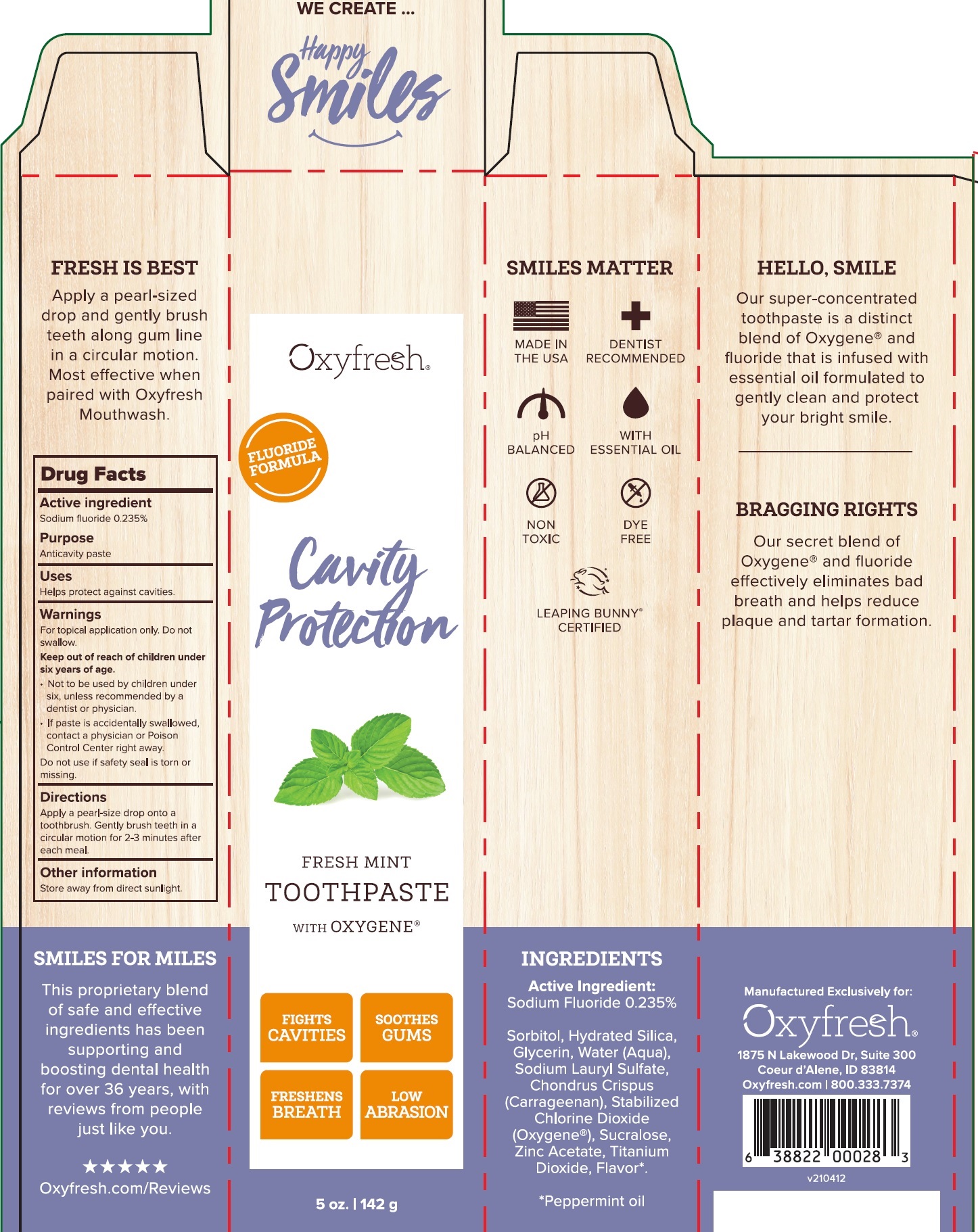
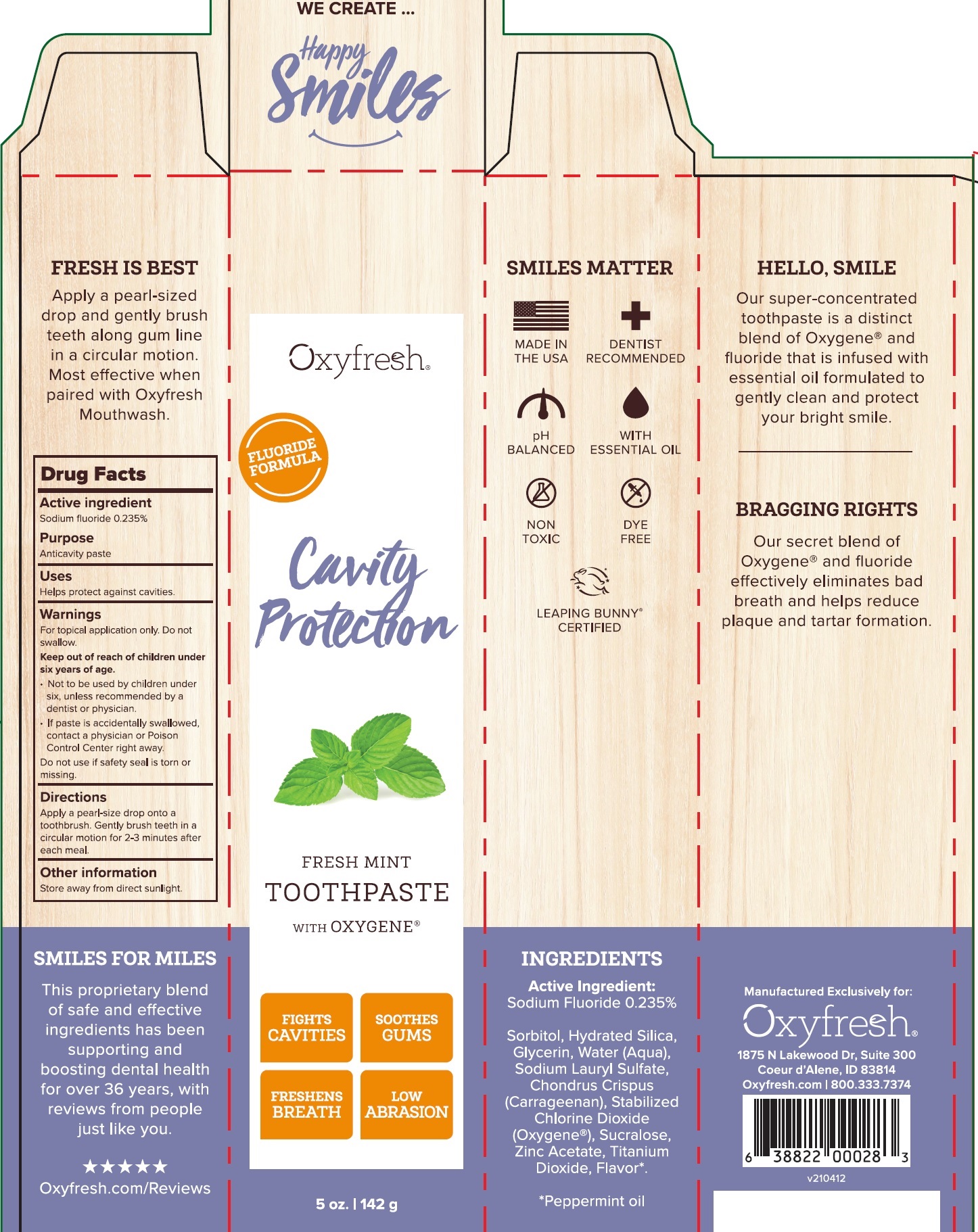
Label: CAVITY PROTECTION FRESH MINT- sodium fluoride paste, dentifrice
- NDC Code(s): 63615-000-01
- Packager: Oxyfresh Worldwide Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- INGREDIENTs
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
CAVITY PROTECTION FRESH MINT
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63615-000 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 2.35 mg in 1 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) HYDRATED SILICA (UNII: Y6O7T4G8P9) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CHONDRUS CRISPUS (UNII: OQS23HUA1X) OXYGEN (UNII: S88TT14065) SUCRALOSE (UNII: 96K6UQ3ZD4) ZINC ACETATE (UNII: FM5526K07A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEPPERMINT OIL (UNII: AV092KU4JH) CHLORINE DIOXIDE (UNII: 8061YMS4RM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63615-000-01 1 in 1 CARTON 05/23/2014 1 142 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 05/23/2014 Labeler - Oxyfresh Worldwide Inc (103385522)