Label: SPF 60 MINERAL SUNSCREEN- titanium dioxide, zinc oxide stick
- NDC Code(s): 72839-547-01, 72839-547-11
- Packager: Derma Care Research Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
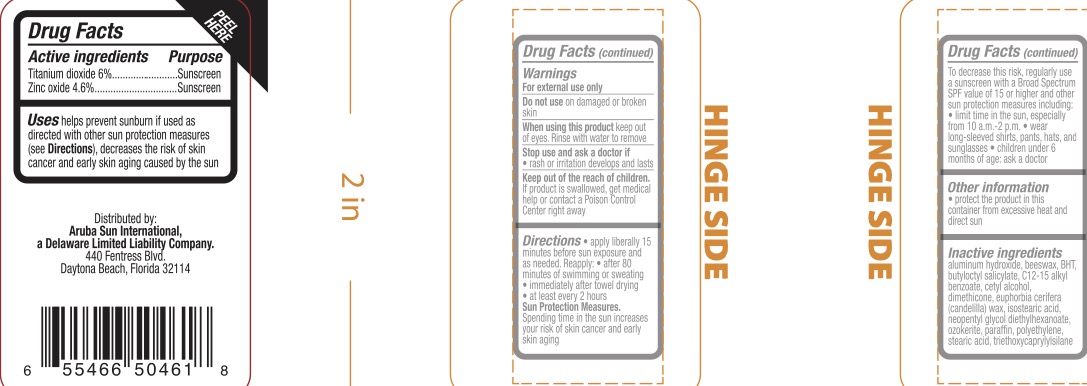
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Apply liberally 15 minutes before sun exposure and as needed. Reapply
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10:00 a.m. - 2:00 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: ask a doctor.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SPF 60 MINERAL SUNSCREEN
titanium dioxide, zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72839-547 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 6 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 4.6 g in 100 g Inactive Ingredients Ingredient Name Strength CANDELILLA WAX (UNII: WL0328HX19) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) CERESIN (UNII: Q1LS2UJO3A) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE 200 (UNII: RGS4T2AS00) ISOSTEARIC ACID (UNII: X33R8U0062) PARAFFIN (UNII: I9O0E3H2ZE) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72839-547-01 14 g in 1 CYLINDER; Type 0: Not a Combination Product 05/23/2023 2 NDC:72839-547-11 14 g in 1 CYLINDER; Type 0: Not a Combination Product 05/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/23/2023 Labeler - Derma Care Research Labs, LLC (116817470) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(72839-547)