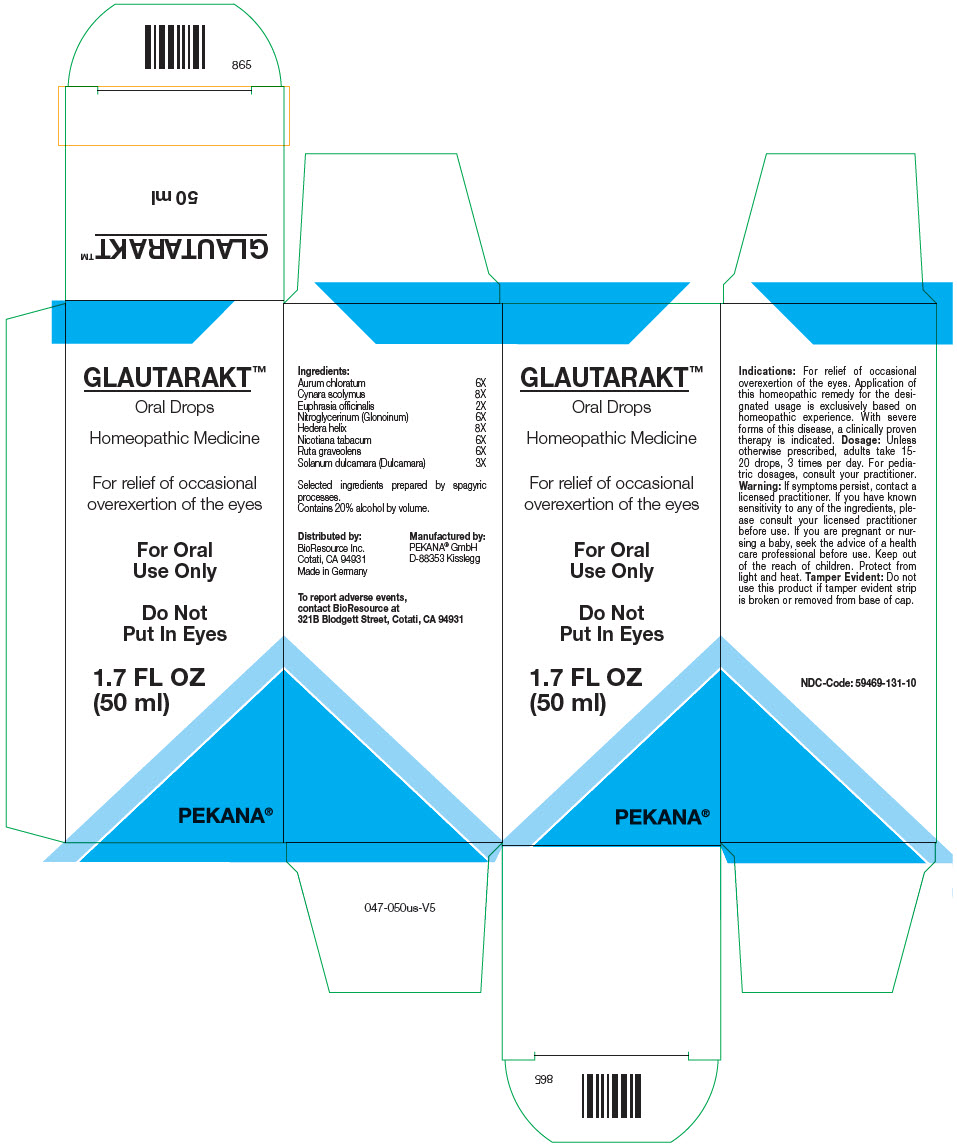
Label: GLAUTARAKT- gold trichloride, solanum dulcamara top, euphrasia stricta, nitroglycerin, hedera helix flowering twig, ruta graveolens flowering top, tobacco leaf, and cynara scolymus whole solution/ drops
- NDC Code(s): 59469-131-10
- Packager: PEKANA Naturheilmittel GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- Indications
- Dosage
- Warning
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ml Bottle Box
-
INGREDIENTS AND APPEARANCE
GLAUTARAKT
gold trichloride, solanum dulcamara top, euphrasia stricta, nitroglycerin, hedera helix flowering twig, ruta graveolens flowering top, tobacco leaf, and cynara scolymus whole solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59469-131 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gold Trichloride (UNII: 15443PR153) (Gold Cation (3+) - UNII:7XM25QYI14) Gold Trichloride 6 [hp_X] in 50 mL Solanum dulcamara Top (UNII: KPS1B1162N) (Solanum Dulcamara Top - UNII:KPS1B1162N) Solanum dulcamara Top 3 [hp_X] in 50 mL Euphrasia Stricta (UNII: C9642I91WL) (Euphrasia Stricta - UNII:C9642I91WL) Euphrasia Stricta 2 [hp_X] in 50 mL Nitroglycerin (UNII: G59M7S0WS3) (Nitroglycerin - UNII:G59M7S0WS3) Nitroglycerin 6 [hp_X] in 50 mL Hedera helix Flowering Twig (UNII: 3D10KUA6BM) (Hedera helix Flowering Twig - UNII:3D10KUA6BM) Hedera helix Flowering Twig 8 [hp_X] in 50 mL Ruta graveolens Flowering Top (UNII: N94C2U587S) (Ruta graveolens Flowering Top - UNII:N94C2U587S) Ruta graveolens Flowering Top 6 [hp_X] in 50 mL Tobacco Leaf (UNII: 6YR2608RSU) (Tobacco Leaf - UNII:6YR2608RSU) Tobacco Leaf 6 [hp_X] in 50 mL Cynara scolymus Whole (UNII: 9N3437ZUU0) (Cynara Scolymus Whole - UNII:9N3437ZUU0) Cynara scolymus Whole 8 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59469-131-10 1 in 1 BOX 12/11/2008 1 50 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 12/11/2008 Labeler - PEKANA Naturheilmittel GmbH (320344542) Establishment Name Address ID/FEI Business Operations PEKANA Naturheilmittel GmbH 320344542 MANUFACTURE(59469-131)