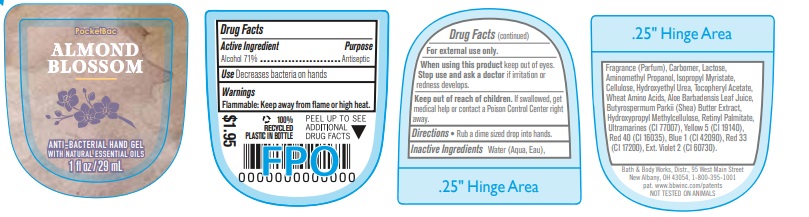
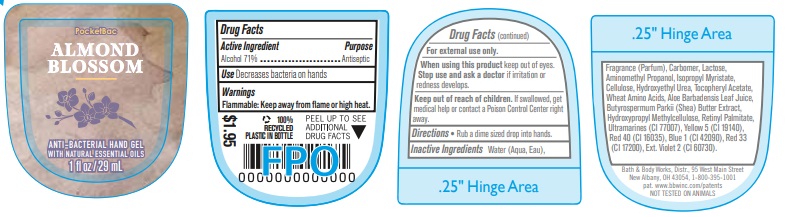
Label: ANTI BACTERIAL HAND GEL ALMOND BLOSSOM- alcohol gel
- NDC Code(s): 62670-6591-0
- Packager: Bath & Body Works, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: Water (Aqua, Eau), Fragrance (Parfum), Carbomer, Lactose, Aminomethyl Propanol, Isopropyl Myristate, Cellulose, Hydroxyethyl Urea, Tocopheryl Acetate, Wheat Amino Acids, Aloe Barbadensis Leaf Juice, Butyrospermum Parkii (Shea) Butter Extract, Hydroxypropyl Methylcellulose, Retinyl Palmitate, Ultramarines (CI 77007), Yellow 5 (CI 19140), Red 40 (CI 16035), Blue 1 (CI 42090), Red 33 (CI 17200), Ext. Violet 2 (CI 60730).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI BACTERIAL HAND GEL ALMOND BLOSSOM
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62670-6591 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 71 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62670-6591-0 29 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2023 10/23/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/23/2023 10/23/2025 Labeler - Bath & Body Works, Inc. (878952845) Establishment Name Address ID/FEI Business Operations KDC US Holdings, Inc. 080783283 manufacture(62670-6591) Establishment Name Address ID/FEI Business Operations Memphis Contract Packaging 117443103 manufacture(62670-6591)