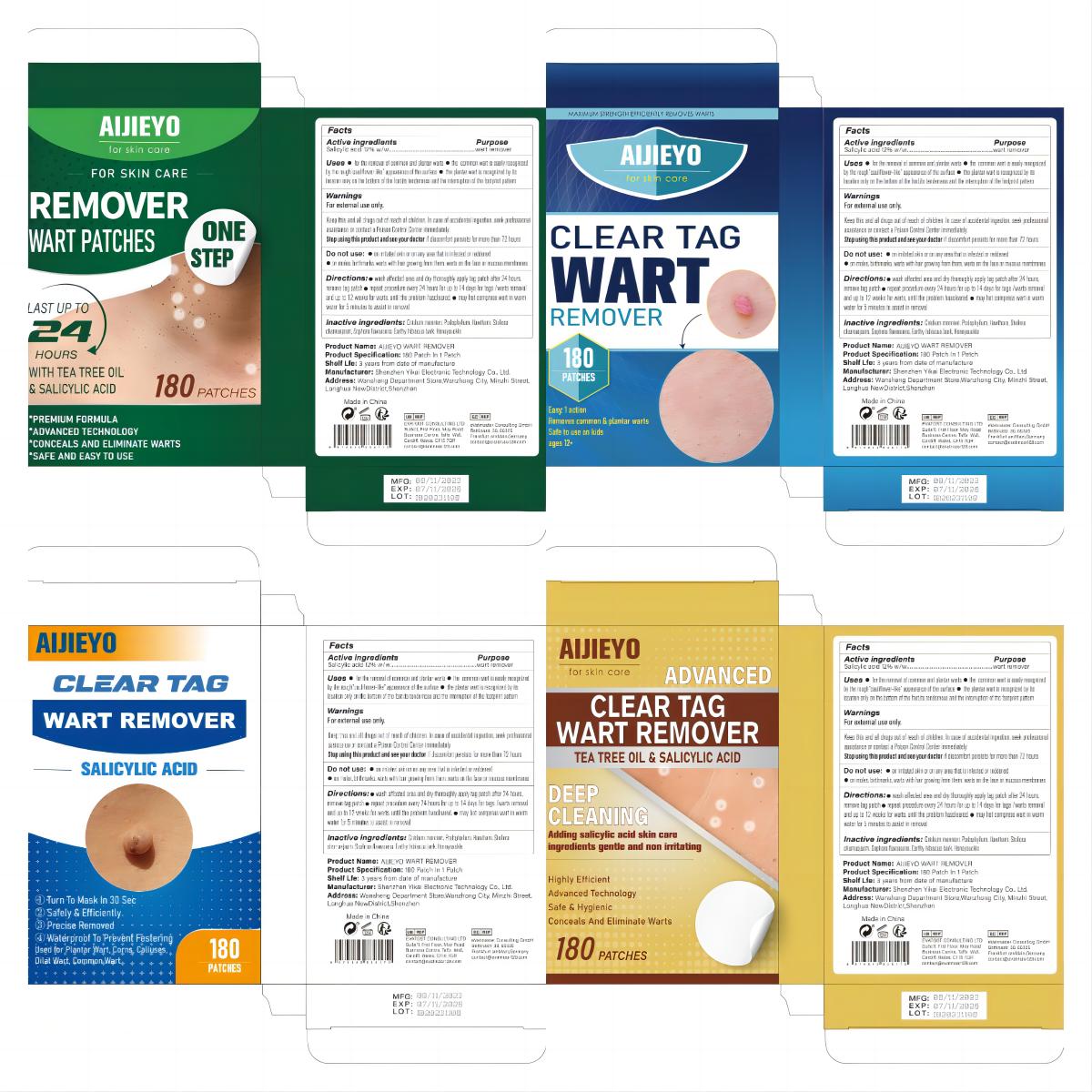
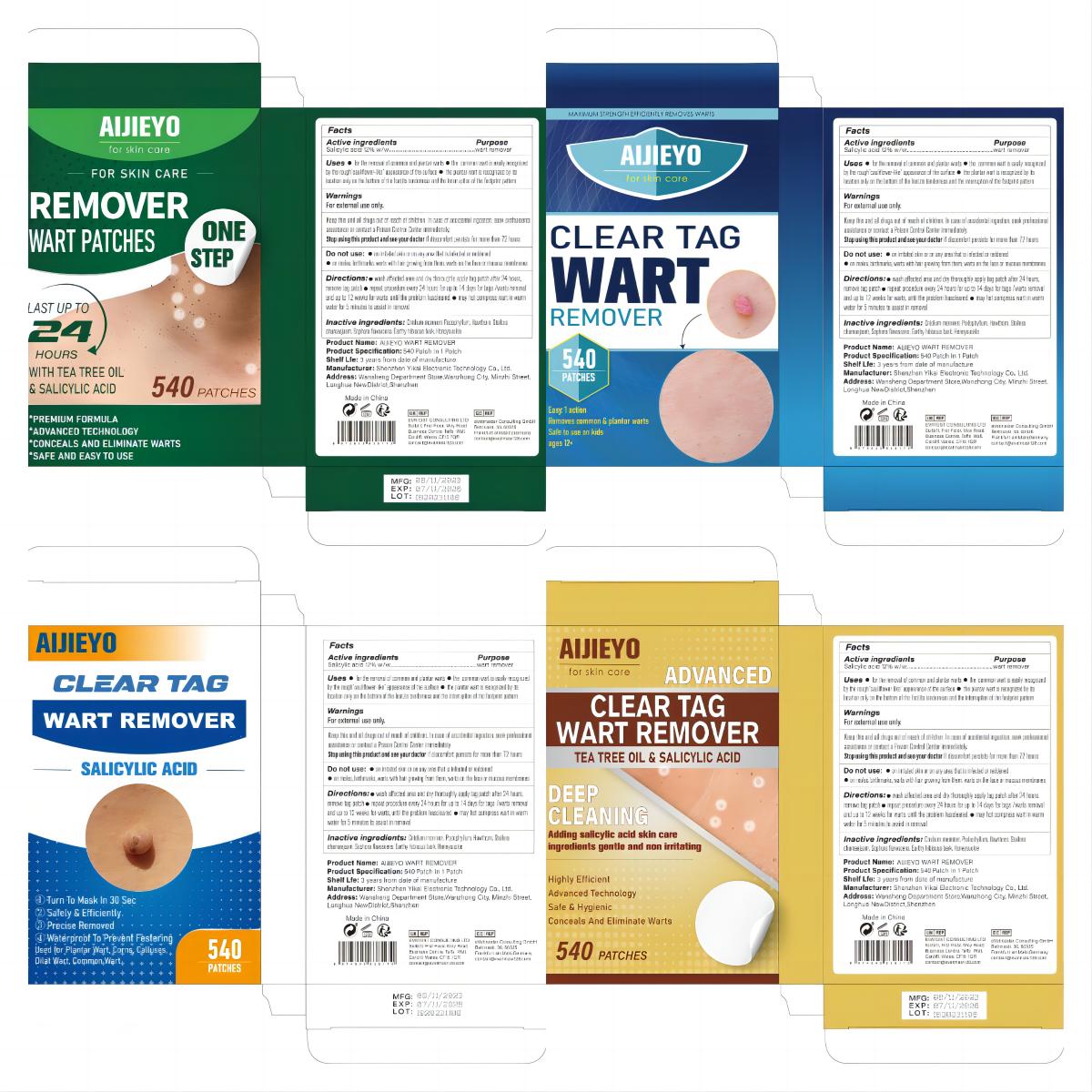
Label: ALJIEYO WART REMOVER patch
- NDC Code(s): 83809-003-01, 83809-003-02
- Packager: Shenzhen Yikai Electronic Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- Keep Oot Of Reach Of Children
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALJIEYO WART REMOVER
aljieyo wart remover patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83809-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 12 g in 100 Inactive Ingredients Ingredient Name Strength PODOPHYLLUM (UNII: 2S713A4VP3) HAWTHORN LEAF WITH FLOWER (UNII: 6OM09RPY36) STELLERA CHAMAEJASME WHOLE (UNII: 80N0P0DD9J) HIBISCUS SYRIACUS BARK (UNII: U6PQI719P3) LONICERA HYPOGLAUCA FLOWER (UNII: 3ADI26ATFY) CNIDIUM MONNIERI FRUIT OIL (UNII: JK0MS9P8YL) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83809-003-01 180 in 1 BOX; Type 0: Not a Combination Product 11/20/2023 2 NDC:83809-003-02 540 in 1 BOX; Type 0: Not a Combination Product 11/20/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 11/20/2023 Labeler - Shenzhen Yikai Electronic Technology Co., Ltd. (700426808) Establishment Name Address ID/FEI Business Operations Shenzhen Yikai Electronic Technology Co., Ltd. 700426808 label(83809-003) , manufacture(83809-003)