Label: ALLERGY- chlorpheniramine maleate tablet
- NDC Code(s): 41163-194-08
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
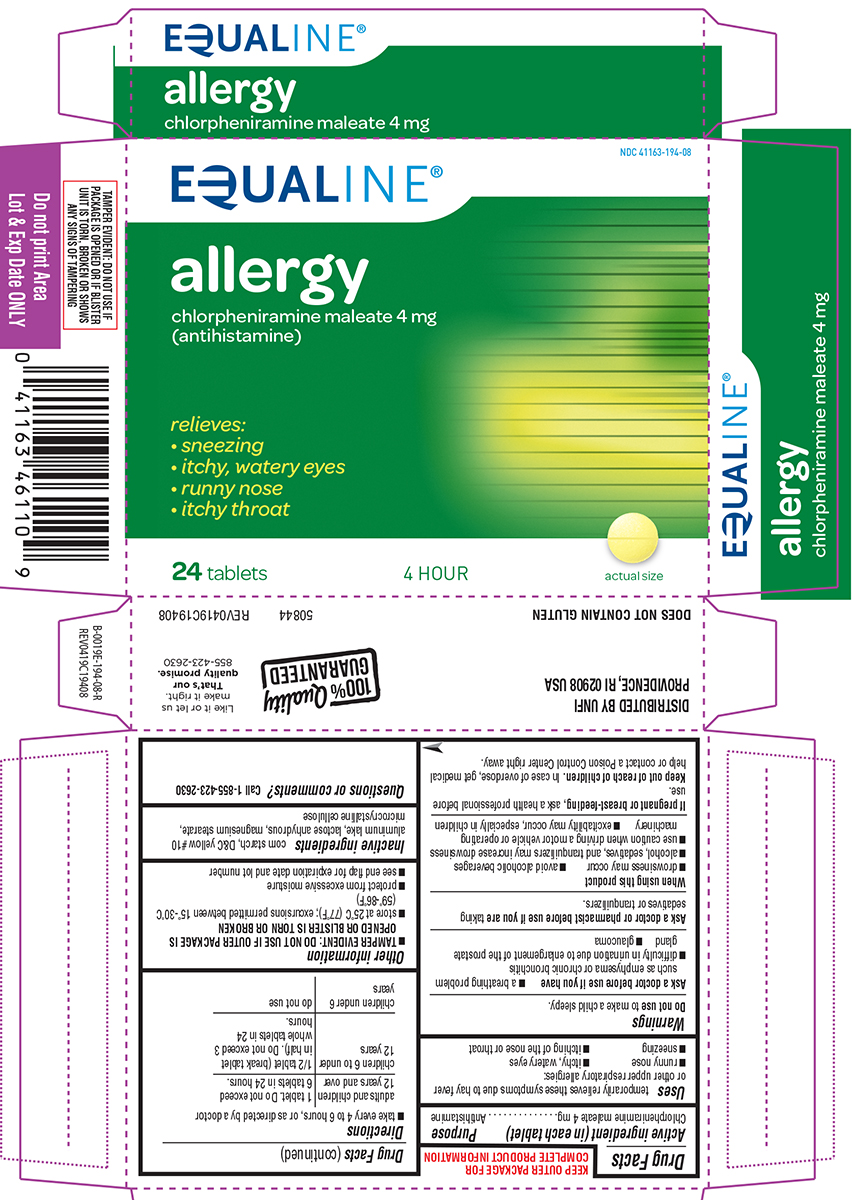
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
EQUALINE®
NDC 41163-194-08
allergy
chlorpheniramine maleate 4 mg
(antihistamine)relieves:
• sneezing
• itchy, watery eyes
• runny nose
• itchy throat24 tablets 4 HOUR actual size
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING50844 REV0419C19408
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USADOES NOT CONTAIN GLUTEN
100% Quality GUARANTEED
Like it or let us
make it right.
That's our
quality promise.
855-423-2630
44-194
-
INGREDIENTS AND APPEARANCE
ALLERGY
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-194 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color yellow Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 44;194 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-194-08 2 in 1 CARTON 12/19/1992 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/19/1992 Labeler - United Natural Foods, Inc. dba UNFI (943556183) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(41163-194) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(41163-194) , pack(41163-194) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(41163-194) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(41163-194) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(41163-194)