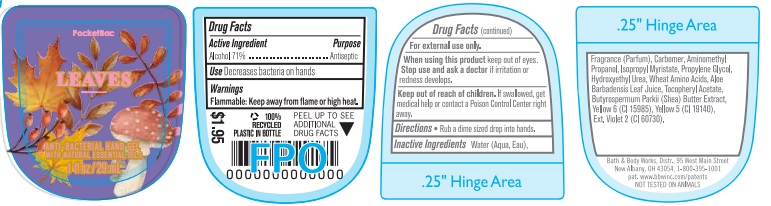
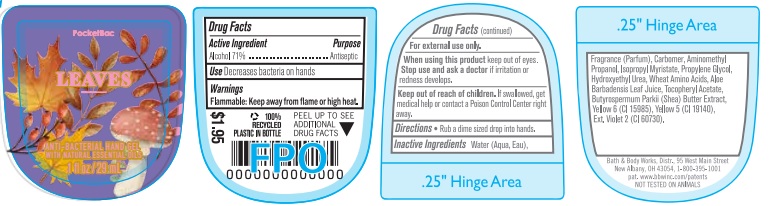
Label: ANTI-BACTERIAL HAND GEL LEAVES- alcohol gel
- NDC Code(s): 62670-6574-0
- Packager: Bath & Body Works, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: Water (Aqua, Eau), Fragrance (Parfum), Carbomer, Aminomethyl Propanol, Isopropyl Myristate, Propylene Glycol, Hydroxyethyl Urea, Wheat Amino Acids, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Butyrospermum Parkii (Shea) Butter Extract, Yellow 6 (CI 15985), Yellow 5 (CI 19140), Ext. Violet 2 (CI 60730).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI-BACTERIAL HAND GEL LEAVES
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62670-6574 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 71 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62670-6574-0 29 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/28/2023 08/28/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/28/2023 08/28/2025 Labeler - Bath & Body Works, Inc. (878952845) Establishment Name Address ID/FEI Business Operations KDC US Holdings, Inc. 080783283 manufacture(62670-6574) Establishment Name Address ID/FEI Business Operations Memphis Contract Packaging 117443103 manufacture(62670-6574)