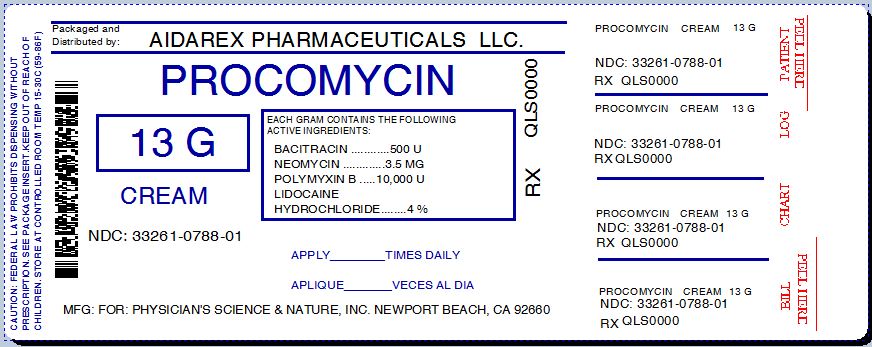
Label: PROCOMYCIN- bacitracin, neomycin, polymyxin b and lidocain hydrochloride cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 33261-788-01 - Packager: Aidarex Pharmaceuticals LLC
- This is a repackaged label.
- Source NDC Code(s): 27495-010
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 28, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Consult a doctor
- Stop use and consult a doctor
- Keep out of reach of children.
- Directions for use
-
Inactive ingredients
acrylates/dimethicone coploymer, allantoin, aloe barbadensis (aloe vera) leaf juice, arachidyl alcohol, arachidyl glucoside, arnica montana flower extract, ascorbic acid (vitamin C), behenyl alcohol, beta-glucan, benzalkonium chloride, beta-glucan, butyrospermum parkii (shea) butter, C 12-15 alkyl benzoate, caprylic/capric triglyceride, cetearyl alcohol, cetearyl glucoside, cyclopentasiloxane, dimethicone, dipalmitoyl hydroxyproline, glycerin, glycine soja (soybean) oil, helianthus annuus (sunflower) seed oil, hydrolyzed mytilus edulis byssus, methylparaben, panthenol, persea gratissima (avocado) oil, phenoxyethanol, polyglyceryl-6 distearate, propylparaben, sclerotium gum, tocopherol, water (aqua)
Manufactured in the USA for:
Physician's Science and Nature, Inc.
220 Newport Center Drive, 11-634,
Newport Beach, CA 92660
Repackaged By:
Aidarex Pharmaceuticals, LLC.
Corona, CA 92880 - Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PROCOMYCIN
bacitracin, neomycin, polymyxin b and lidocain hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:33261-788(NDC:27495-010) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN (UNII: 58H6RWO52I) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [iU] in 1 g NEOMYCIN (UNII: I16QD7X297) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 0.0035 g in 1 g POLYMYXIN B (UNII: J2VZ07J96K) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [iU] in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) ASCORBIC ACID (UNII: PQ6CK8PD0R) DOCOSANOL (UNII: 9G1OE216XY) ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) GLYCERIN (UNII: PDC6A3C0OX) SOYBEAN (UNII: L7HT8F1ZOD) SUNFLOWER OIL (UNII: 3W1JG795YI) METHYLPARABEN (UNII: A2I8C7HI9T) PANTHENOL (UNII: WV9CM0O67Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33261-788-01 15 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 10/01/2010 Labeler - Aidarex Pharmaceuticals LLC (801503249)