Label: ACNE CARE LOTN- salicylic acid lotion
- NDC Code(s): 68062-2267-1
- Packager: Spa de Soleil
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
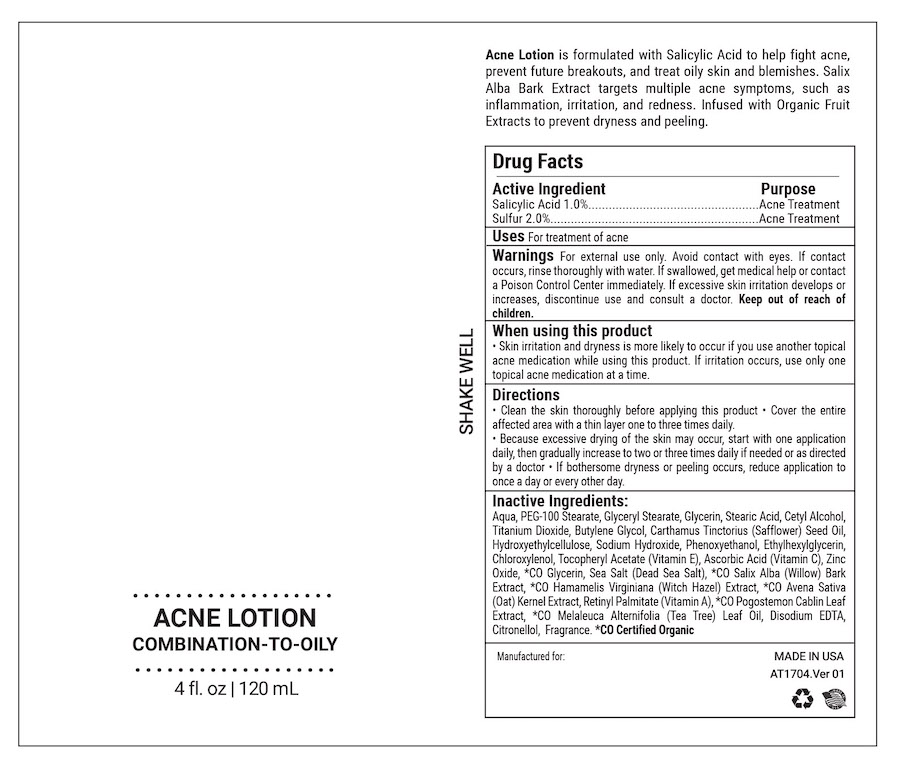
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
If swallowed, get medical help or contact a Poison Control Center right away. If excessive skin irritation develops or increases, discontinue use and consult a doctor. Keep out of reach of children.
- KEEP OUT OF REACH OF CHILDREN
- WHEN USING
-
DOSAGE & ADMINISTRATION
Directions
• Clean the skin thoroughly before applying this product • Cover the entire affected area with a thin layer one to three times daily.
• Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor • If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENT
Other Ingredients:
Aqua, PEG – 100 Stearate, Glyceryl Stearate, Glycerin, Stearic Acid, Cetyl Alcohol, Titanium Dioxide, Butylene Glycol, Carthamus Tinctorius (Safflower) Seed Oil, Zinc Oxide, Hydroxyethyl Cellulose, Sodium Hydroxide, Phenoxyethanol, Ethylhexylglycerin, Chloroxylenol, Tocopheryl Acetate (Vitamin E), Ascorbic Acid (Vitamin C), Malus Domestica (Apple) Fruit Cell Culture Extract, Citric Acid, Lactic Acid, Malic Acid, Gluconic Acid, Glycolic Acid, Salicylic Acid, Tartaric Acid, *CO Alcohol, *CO Glycerin, *CO Salix Alba Bark Extract, *CO Avena Sativa (Oat) Kernel Extract, *CO Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, *CO Pogostemon Cablin Oil, *CO Melaleuca Alternifolia (Tea Tree) Leaf Oil, Disodium EDTA, Sea Salt, **Squalane, Fragrance.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE CARE LOTN
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68062-2267 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 mg in 100 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL STEARATE/PEG-100 STEARATE (UNII: RD25J5V947) GLYCERIN (UNII: PDC6A3C0OX) STEARIC ACID D7 (UNII: T3B081197X) CETYL ALCOHOL (UNII: 936JST6JCN) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68062-2267-1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/19/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/19/2023 Labeler - Spa de Soleil (874682867) Registrant - Spa de Soleil (874682867) Establishment Name Address ID/FEI Business Operations Spa de Soleil 874682867 manufacture(68062-2267)