Label: ANTISEPTIC MOUTH SORE- hydrogen peroxide, menthol rinse
- NDC Code(s): 69842-515-43
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
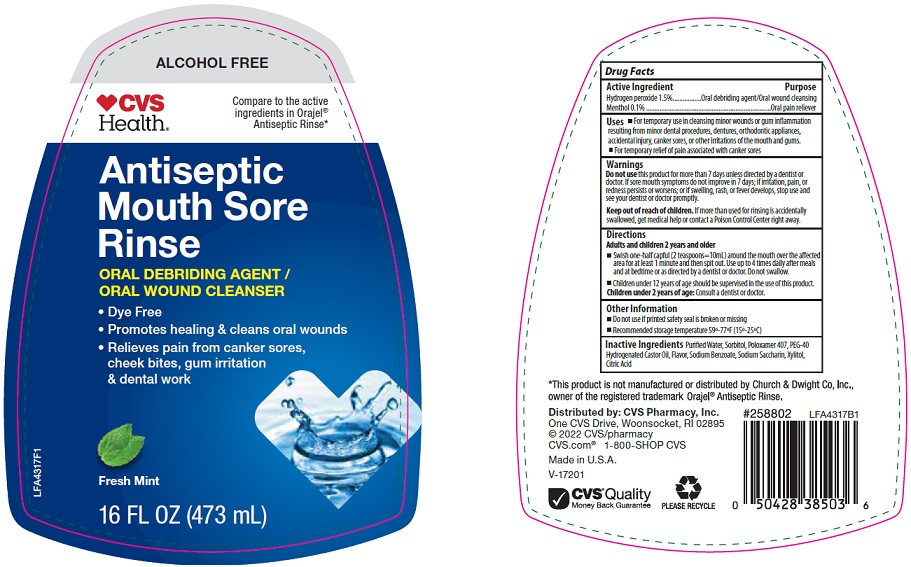
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
Adults and children 2 years and older
- •
- Swish one-half capful (2 teaspoons=10mL) around the mouth over the affected area for at least 1 minute and then spit out. Use up to 4 times daily after meals and at bedtime or as directed by a dentist or doctor. Do not swallow.
- •
- Children under 12 years of age should be supervised in the use of this product.
Children under 2 years of age: Consult a dentist or doctor.
- Other Information
- Inactive Ingredients
-
Package/Label Principal Display Panel
ALCOHOL FREE
CVS Health®
Compare to the active ingredients in Orajel® Antiseptic Rinse
Antiseptic Mouth Sore Rinse
ORAL DEBRIDING AGENT / ORAL WOUND CLEANSER
- Dye Free
- Promotes healing & cleans oral wounds
- Relieves pain from canker sores, cheek bites, gum irritation & dental work
Fresh Mint
16 FL OZ (473 mL)
LFA4317F1
-
INGREDIENTS AND APPEARANCE
ANTISEPTIC MOUTH SORE
hydrogen peroxide, menthol rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-515 Route of Administration PERIODONTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) POLOXAMER 407 (UNII: TUF2IVW3M2) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) SODIUM BENZOATE (UNII: OJ245FE5EU) SACCHARIN SODIUM (UNII: SB8ZUX40TY) XYLITOL (UNII: VCQ006KQ1E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-515-43 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/18/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/18/2021 Labeler - CVS Pharmacy (062312574)