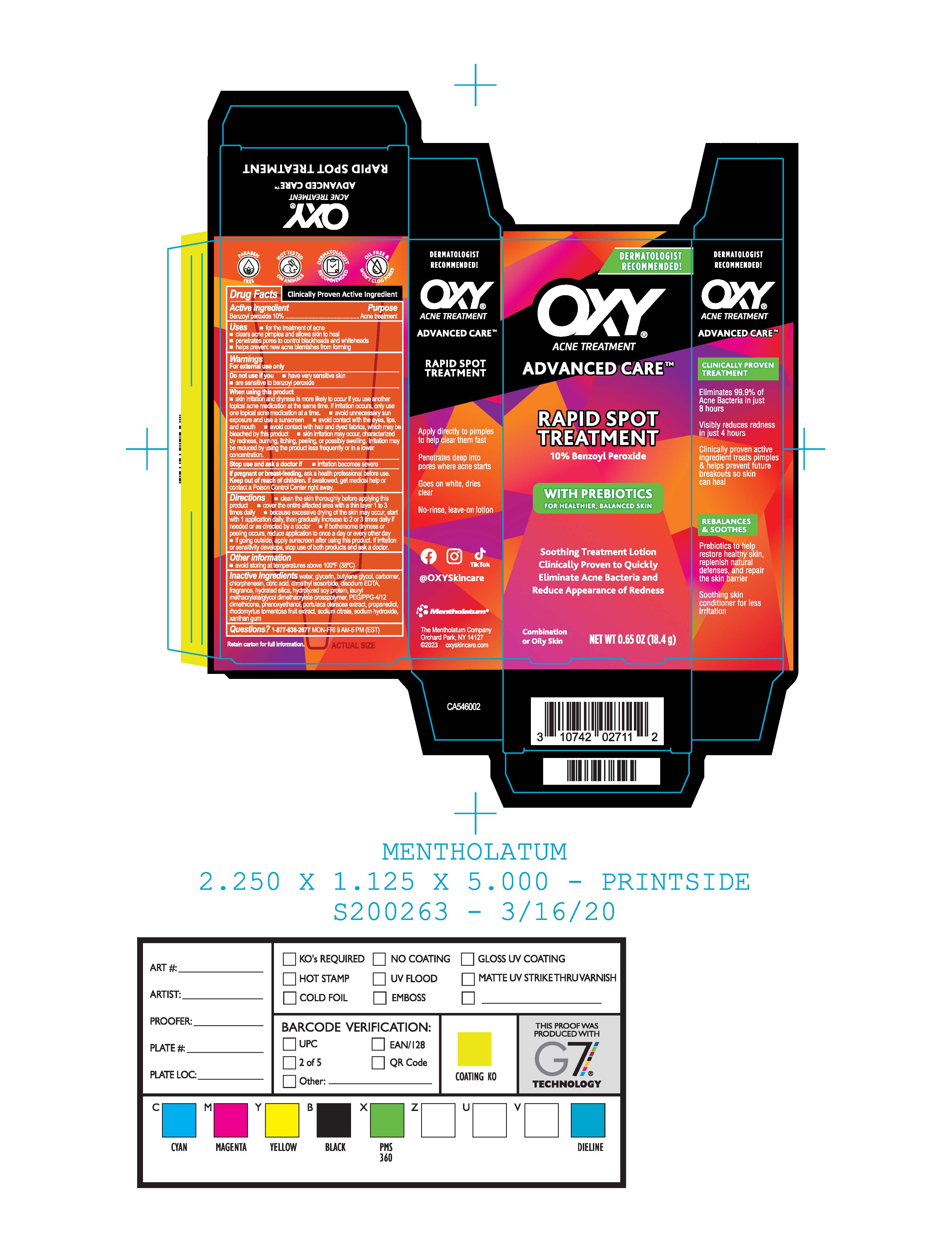
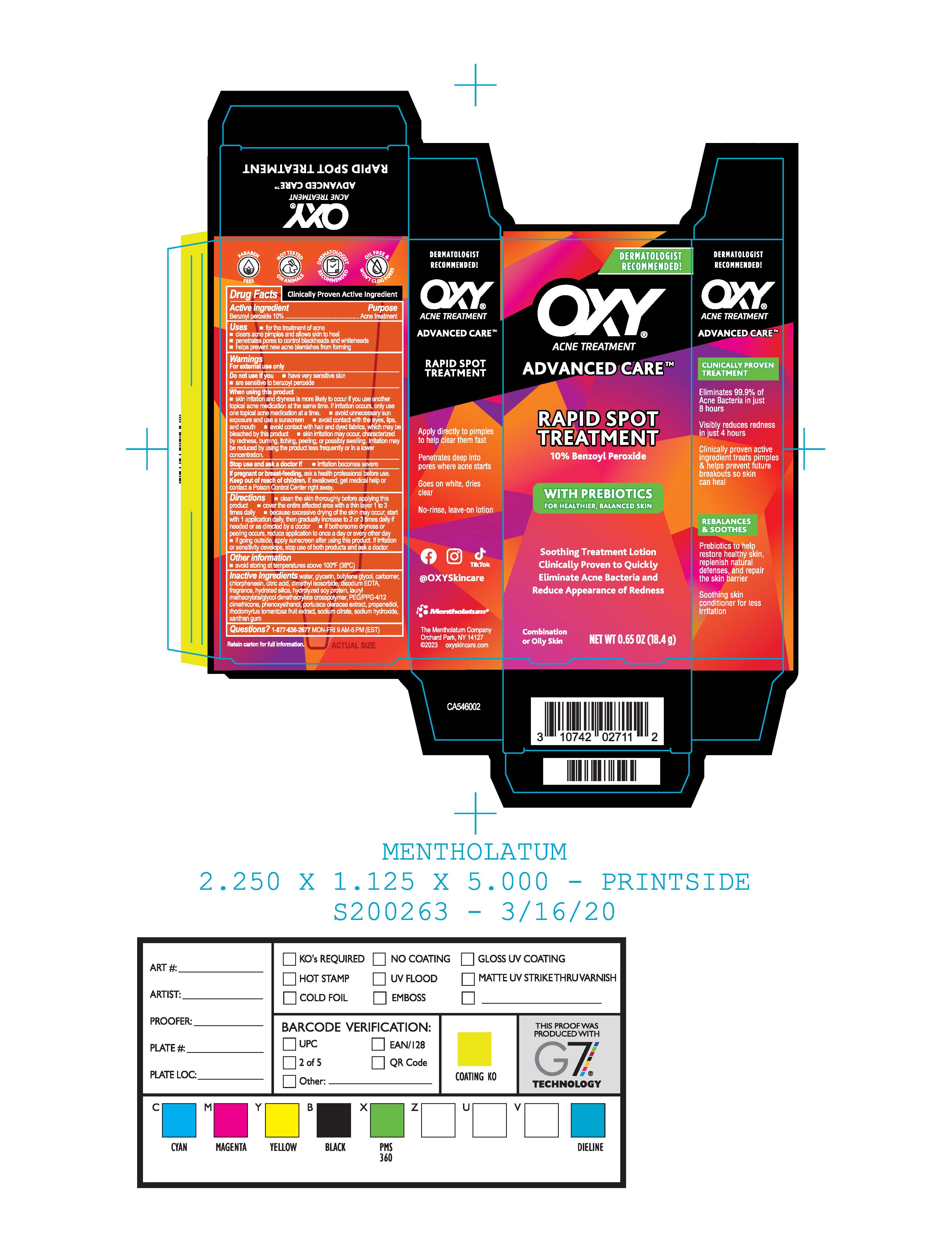
Label: OXY ADVANCED CARE RAPID SPOT TREATMENT- benzoyl peroxide gel
- NDC Code(s): 10742-1201-1, 10742-1201-2, 10742-1201-3
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
- Keep out of reach of children.
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer 1 to 3 times daily
- because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
-
Inactive Ingredients
water, glycerin, butylene glycol, carbomer, chlorphenesin, citric acid, dimethyl isosorbide, disodium EDTA, fragrance, hydrated silica, hydrolyzed soy protein, lauryl methacrylate/glycol dimethacrylate crosspolymer, PEG/PPG-4/12 dimethicone, phenoxyethanol, portulaca oleracea extract, propanediol, rhodomyrtus tomentosa fruit extract, sodium citrate, sodium hydroxide, xanthan gum
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
OXY ADVANCED CARE RAPID SPOT TREATMENT
benzoyl peroxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-1201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CHLORPHENESIN (UNII: I670DAL4SZ) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDRATED SILICA (UNII: Y6O7T4G8P9) SOY PROTEIN (UNII: R44IWB3RN5) LAURYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EX0F4CZ66H) PEG/PPG-4/12 DIMETHICONE (UNII: JAN3585W85) PHENOXYETHANOL (UNII: HIE492ZZ3T) PURSLANE (UNII: M6S840WXG5) PROPANEDIOL (UNII: 5965N8W85T) RHODOMYRTUS TOMENTOSA FRUIT (UNII: Q99511S58K) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-1201-1 1 in 1 CARTON 07/12/2021 1 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10742-1201-2 1 in 1 CARTON 12/01/2021 2 32.6 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:10742-1201-3 1 in 1 CARTON 02/21/2022 3 18.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/12/2021 Labeler - The Mentholatum Company (002105757)