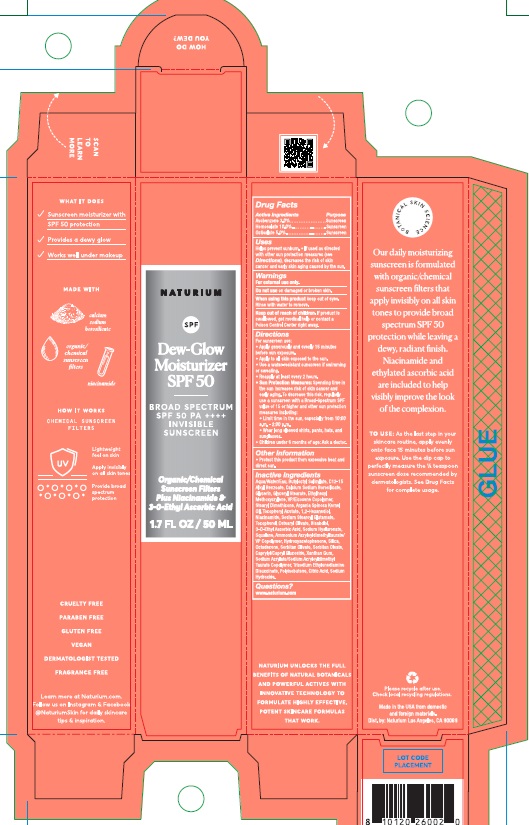
Label: NATURIUM DEW-GLOW MOISTURIZER SPF 50- homosalate, octisalate, avobenzone lotion
- NDC Code(s): 76354-130-01, 76354-130-02, 76354-130-03
- Packager: e.l.f. Cosmetics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
For sunscreen use:
- Apply generously and evenly 15 minutes before sun exposure.
- Apply to all skin exposed to the sun.
- Use a water-resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Sun Protection Measures: Spending time in the sun increases risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10:00 a.m. - 2:00 p.m.
- Wear long sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age: Ask a doctor.
- Other Information
-
Inactive Ingredients
Aqua/Water/Eau, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Calcium Sodium Borosilicate, Glycerin, Glyceryl Stearate, Ethylhexyl Methoxycrylene, VP/Eicosene Copolymer, Stearyl Dimethicone, Argania Spinosa Kernel Oil, Tocopheryl Acetate, 1,2-Hexandiol, Niacinamide, Sodium Stearoyl Glutamate, Tocopherol, Cetearyl Olivate, Bisabolol, 3-O-Ethyl Ascorbic Acid, Sodium Hyaluronate, Squalane, Ammonium Acryloyldimethyltaurate/VP Copolymer, Hydroxyacetophnone, Silica, Octadecene, Sorbitan Olivate, Sorbitan Oleate, Caprylyl/Capryl Glucoside, Xanthan Gum, Sodium Acrylate/Sodium Acryloyldimethyltaurate/VP Copolymer, Hydroxyacetophenone, Silica, Octadecene, Sorbitan Olivate, Sorbitan Oleate, Caprylyl/Capryl Glucoside, Xanthan Gum, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Trisodium Ethylenediamine Disuccinate, Polyisobutene, Citric Acid, Sodium Hydroxide.
- Questions?
- Product Packaging
-
INGREDIENTS AND APPEARANCE
NATURIUM DEW-GLOW MOISTURIZER SPF 50
homosalate, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76354-130 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BOROSILICATE GLASS (UNII: BOJ6T9AR90) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SQUALANE (UNII: GW89575KF9) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) LEVOMENOL (UNII: 24WE03BX2T) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) NIACINAMIDE (UNII: 25X51I8RD4) CETEARYL OLIVATE (UNII: 58B69Q84JO) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) STEARYL DIMETHICONE (400 MPA.S AT 50C) (UNII: R327X197HY) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN OLIVATE (UNII: MDL271E3GR) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) OCTADECENE (UNII: H5ZUQ6V4AK) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76354-130-01 1 in 1 CARTON 10/24/2022 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:76354-130-02 1 in 1 CARTON 12/27/2023 2 100 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:76354-130-03 1 in 1 CARTON 12/27/2023 3 20 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/24/2022 Labeler - e.l.f. Cosmetics, Inc (093902816)