Label: NOVOEIGHT- antihemophilic factor recombinant kit
-
NDC Code(s):
0169-7008-98,
0169-7810-01,
0169-7811-11,
0169-7815-01, view more0169-7820-01, 0169-7821-11, 0169-7825-01, 0169-7829-11, 0169-7830-01, 0169-7831-11, 0169-7850-01, 0169-7851-11, 0169-7855-11
- Packager: Novo Nordisk
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 31, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NOVOEIGHT safely and effectively. See full prescribing information for NOVOEIGHT.
NOVOEIGHT (antihemophilic factor, recombinant)
lyophilized powder for solution, for intravenous use
Initial U.S. Approval: 2013INDICATIONS AND USAGE
Novoeight is an Antihemophilic Factor (Recombinant) indicated for use in adults and children with hemophilia A for:
- •
- On-demand treatment and control of bleeding episodes
- •
- Perioperative management
- •
- Routine prophylaxis to reduce the frequency of bleeding episodes.
Novoeight is not indicated for the treatment of von Willebrand disease. (1)
DOSAGE AND ADMINISTRATION
For intravenous injection after reconstitution only (2)
- •
- Each vial of Novoeight contains the labeled amount of recombinant Factor VIII in international units (IU). (2)
- •
- The required dosage is determined using the following formula:
- Dosage Required (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL)
- •
- Frequency of Novoeight administration is determined by the type of bleeding episode and the recommendation of the treating physician. (2.1)
DOSAGE FORMS AND STRENGTHS
Novoeight is available as a lyophilized powder in single-dose vials of 250, 500, 1000, 1500, 2000 and 3000 international units. (3)
CONTRAINDICATIONS
Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis, to Novoeight or its components, including hamster proteins. (4)
WARNINGS AND PRECAUTIONS
- •
- Anaphylaxis and severe hypersensitivity reactions are possible. Patients may develop hypersensitivity to hamster proteins, which are present in trace amounts in the product. Should symptoms occur, discontinue Novoeight and administer appropriate treatment. (5.1)
- •
- Development of activity-neutralizing antibodies (inhibitors) may occur. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor VIII inhibitor concentration. (5.2, 5.3)
ADVERSE REACTIONS
The most frequently reported adverse reactions (≥ 1%) were inhibitors in Previously Untreated Patients (PUPs), injection site reactions, and pyrexia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-844-303-4448 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- •
- Pediatric Use: Clearance (based on per kg body weight) is higher in children. Higher or more frequent dosing may be needed. (8.4)
- •
- Obesity: The area under the curve (AUC) is higher and clearance lower in adult patients with body mass index (BMI) ≥ 30 kg/m2 than in patients with BMI < 30kg/m2. Adjust dose as necessary. (8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Neutralizing Antibodies
5.3 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Obesity
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Novoeight, Antihemophilic Factor (Recombinant), is a human antihemophilic factor (human blood coagulation
factor VIII (FVIII)) indicated for use in adults and children with hemophilia A for:
- •
- On-demand treatment and control of bleeding episodes
- •
- Perioperative management
- •
- Routine prophylaxis to reduce the frequency of bleeding episodes
Novoeight is not indicated for the treatment of von Willebrand disease.
-
2 DOSAGE AND ADMINISTRATION
For intravenous injection after reconstitution only.
2.1 Dose
- •
- Dosage and duration of treatment depend on the severity of the factor VIII deficiency, on the location and extent of bleeding, and the patient’s clinical condition. Careful monitoring of replacement therapy is necessary in cases of major surgery or life-threatening bleeding episodes.
- •
- Each vial of Novoeight contains the labeled amount of recombinant factor VIII in international units (IU). One IU of factor VIII activity corresponds to the quantity of factor VIII in one milliliter of normal human plasma. The calculation of the required dosage of factor VIII is based on the empirical finding that one IU of factor VIII per kg body weight raises the plasma factor VIII activity by two IU/dL. This relationship causes a factor of 0.5 to be present in the dose calculation formula shown below.
- •
- The required dosage can be determined using the following formula:
- Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5
The final dose calculated is expressed as IU
- •
- Base the dose and frequency of Novoeight on the individual clinical response. Patients may vary in their pharmacokinetic and clinical responses [See Clinical Pharmacology (12.3)].
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing Novoeight for on-demand treatment and control of bleeding episodes is provided in Table 1. Dose to maintain a plasma factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 1.
Table 1: Dosing for On-demand Treatment and Control of Bleeding Episodes
Type of Bleeding Episodes
Factor VIII Level Required (IU/dL or % of normal)
Frequency of Doses (hours)
Duration of Therapy (days)
Minor
Early hemarthrosis, minor muscle or oral bleeding
20-40
12-24
At least 1 day until bleeding resolution is achieved
Moderate
Muscle bleeding, bleeding into the oral cavity or mild head trauma
30-60
12-24
Until pain and acute disability are resolved (approximately 3-4 days)
Major
Life or limb threatening hemorrhage, Gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, fractures
60-100
8-24
Until resolution of bleed (approximately 7-10 days)
Perioperative Management
A guide for dosing Novoeight during surgery (perioperative management) is provided in Table 2. Consider maintaining a plasma factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 2.
Table 2: Dosing for Perioperative Management
Type of Surgery
Factor VIII Level Required (IU/dL or % of normal)
Frequency of Doses (hours)
Duration of Therapy (days)
Minor
Including tooth extraction
30-60
24
At least 1 day until healing is achieved
Major
Intracranial, intra-abdominal, intrathoracic, or joint replacement surgery
80-100
(pre-and post-operative)
8-24
Until adequate wound healing, then continue therapy for at least 7 days to maintain a factor VIII activity of 30% to 60% (IU/dL)
Routine Prophylaxis
A guide for dosing Novoeight for routine prophylaxis is included below in Table 3.
Table 3: Dosing for Routine Prophylaxis
Patient Population
Factor VIII Dose Required (IU/kg)
Frequency of Doses
Adults and adolescents (≥ 12 years)
20-50
3 times weekly
20-40
Every other day
Children (<12 years)
25-60
3 times weekly
25-50
Every other day
2.2 Preparation and Reconstitution
- •
- Always wash hands and ensure that the area is clean before performing the procedures.
- •
- Use aseptic technique during the reconstitution procedures.
- •
- If the dose requires more than one vial of Novoeight per injection, reconstitute each vial according to the following instructions:
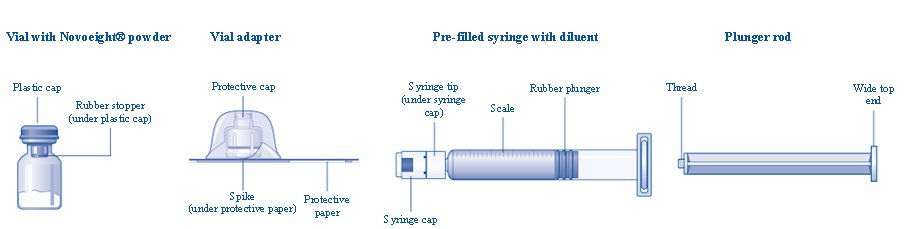
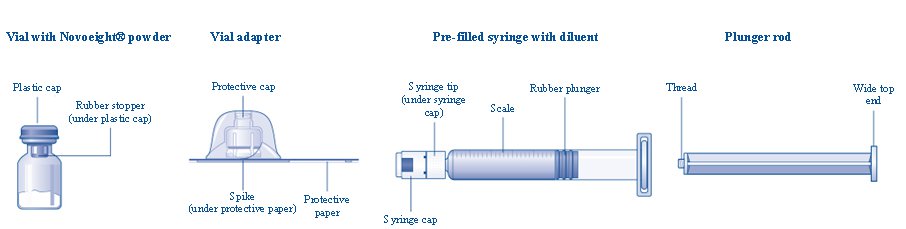
Overview of Novoeight Package
Reconstitution
- 1.
- Bring the Novoeight vial and the pre-filled diluent syringe to room temperature.
- 2.
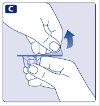
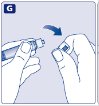
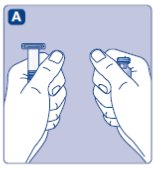
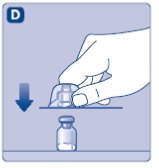
- Remove the plastic cap from the Novoeight vial.
- 3.
- Wipe the rubber stopper on the vial with a sterile alcohol swab and allow it to dry prior to use.
- 4.
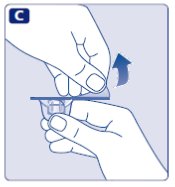
- Remove the protective paper from the vial adapter. Do not remove the vial adapter from the protective cap.
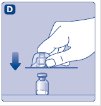
- 5.
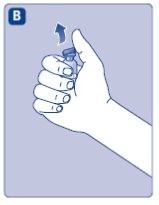
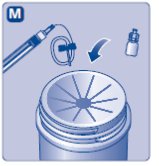
- Place the vial on a flat and solid surface. While holding the protective cap, place the vial adapter over the Novoeight vial and press down firmly on the protective cap until the vial adapter spike penetrates the rubber stopper.
- 6.
- Carefully remove the protective cap from the vial adapter.
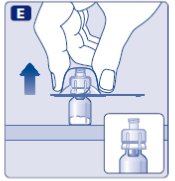
- 7.
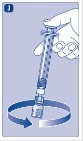
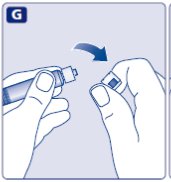
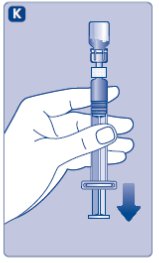
- Grasp the plunger rod as shown in the diagram. Attach the plunger rod to the syringe by holding the plunger rod by the wide top end. Turn the plunger rod clockwise into the rubber plunger inside the pre-filled diluent syringe until resistance is felt.
- 8.
- Break off the syringe cap from the pre-filled diluent syringe by snapping the perforation of the cap.
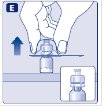
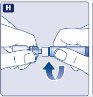
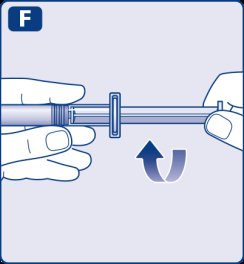
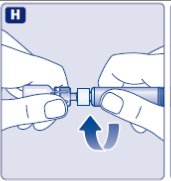
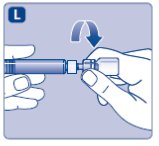
- 9.
- Connect the pre-filled diluent syringe to the vial adapter by turning it clockwise until it is secured.
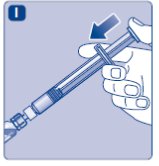
- 10.
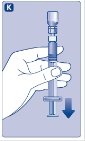
- Push the plunger rod to slowly inject all the diluent into the vial.
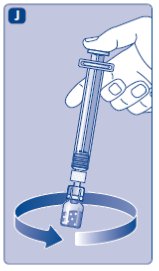
- 11.
- Without removing the syringe, gently swirl the Novoeight vial until all of the powder is dissolved.
- 12.
- Use the Novoeight solution immediately. If not, store the solution in the vial with the vial adapter and the syringe attached. Use Novoeight within 4 hours after reconstitution when stored at <86°F (30°C) or within 2 hours when stored between 86°F (30°C) to 104°F (40°C).
2.3 Administration
For intravenous injection only.
- •
- Inspect the reconstituted Novoeight solution visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
- •
- Do not administer Novoeight in the same tubing or container with other medicinal products.
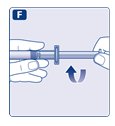
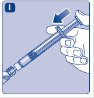
- 1.
- Invert the Novoeight vial and slowly draw the solution into the syringe.
- 2.
- Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- 3.
- Attach the syringe to the luer end of an infusion needle set.
- 4.
- Inject the reconstituted Novoeight intravenously slowly over 2 to 5 minutes.
- 5.
- After injection, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused Novoeight and other waste materials. Accidental needle stick with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs. Place needles in a sharps container after single-use.
Caution:
The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer Novoeight through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
-
3 DOSAGE FORMS AND STRENGTHS
Novoeight is available as a white lyophilized powder in single-dose vials containing 250, 500, 1000, 1500, 2000 and 3000 international units per vial.
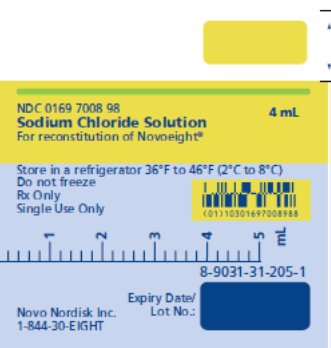
After reconstitution with 4 mL of 0.9% sodium chloride solution, each mL of reconstituted solution contains approximately 62.5, 125, 250, 375, 500 or 750 international units of Novoeight, respectively.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, are possible with Novoeight. Novoeight contains trace amounts of hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins. Early signs of hypersensitivity reactions that can progress to anaphylaxis include angioedema, chest tightness, dyspnea, wheezing, urticaria, and pruritus. Immediately discontinue administration and initiate appropriate treatment if allergic- or anaphylactic-type reactions occur.
5.2 Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to factor VIII can occur following administration of Novoeight. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all factor VIII products. Inhibitors have been reported following administration of Novoeight in PUPs [see Adverse Reactions (6.1)]. Monitor all patients for the development of inhibitors by appropriate clinical observation and laboratory testing. If the expected plasma levels of factor VIII activity are not attained, or if bleeding is not controlled with an appropriate dose, perform testing for factor VIII inhibitors.
5.3 Monitoring Laboratory Tests
- •
- Monitor plasma factor VIII activity levels by the one-stage clotting assay or the chromogenic substrate assay to confirm that adequate factor VIII levels have been achieved and maintained, when clinically indicated. [See Dosage and Administration(2.1)]
- •
- Perform assay to determine if factor VIII inhibitor is present if expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with the expected dose of Novoeight. Determine inhibitor levels in Bethesda Units.
-
6 ADVERSE REACTIONS
The most frequently reported adverse reactions observed in clinical trials (≥ 1%) were injection site reactions, and pyrexia.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
During the clinical development of Novoeight, 301 male patients (242 previously treated patients (PTPs); exposed to a factor VIII-containing product for ≥150 days and 59 Previously Untreated Patients (PUPs)) with severe hemophilia A (factor VIII level ≤1%) received at least one dose of Novoeight as part of either routine prophylaxis, on-demand treatment of bleeding episodes, perioperative management of major and minor surgical, dental, or other invasive procedures, Immune Tolerance Induction (ITI) or pharmacokinetic evaluation of Novoeight with more than 140,000 exposure days (corresponding to over 900 patient years). During prophylaxis treatment subjects received a median of 468 injections of Novoeight (range 1-1317).
Table 4: Summary of Adverse Reactions (ARs ) with a Frequency ≥ 1% in 301 Subjects
- MedDRA System Organ class
- Adverse Reactions
- Frequency N (%)
- General disorders and
- administration site conditions
- Pyrexia
- 3 (1.0%)
- Injection site reaction
- 3 (1.0%)
Immunogenicity
Subjects were monitored for neutralizing antibodies to factor VIII and binding antibodies to CHO and murine protein. No PTPs developed confirmed neutralizing antibodies to factor VIII. One twenty-two month old previously treated child had a positive neutralizing antibody to factor VIII of 1.3 [BU] in the Bethesda assay after 15 exposure days that was not confirmed when checked after 20 exposure days. In vivo recovery was normal for this child and no clinical adverse findings were observed. In the completed main phase of the clinical trial in PUPs, 24 of 56 (42.9%) patients developed inhibitors with a mean of 14.1 exposure days at the time of the first positive inhibitor test; 15 (26.8%) PUPs developed high titer (≥ 5 BU) inhibitors. High risk genetic mutations were identified in 91.7% of the overall inhibitors and 93.3% of the high titer inhibitors.
No patients developed de novo anti-murine antibodies. Nineteen subjects were positive for anti-Chinese hamster ovary (CHO) cell protein antibodies. Two of these subjects changed from anti-CHO negative to anti-CHO positive and 6 subjects changed from anti-CHO positive to anti-CHO negative. The remaining 11 subjects were either positive throughout the trials (n=6), negative at baseline and end-of trial but with transient positive samples (n=2), or positive at baseline and end-of trial but with negative samples in between (n=3). No clinical adverse findings were observed in any of these subjects.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to Novoeight with the incidence of antibodies to other products.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
As hemophilia mainly affects males, there are no adequate and well-controlled studies using Novoeight in pregnant women to determine whether there is a drug-associated risk. Animal reproduction studies have not been conducted with Novoeight.
In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. There is no reliable data on the incidences specific to the hemophilia A population.
8.2 Lactation
Risk Summary
There is no information regarding the presence of Novoeight in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Novoeight and any potential adverse effects on the breastfed infant from Novoeight or from the underlying maternal condition.
8.4 Pediatric Use
Children have shorter half-life and lower recovery of factor VIII than adults. Because clearance (based on per kg body weight) has been demonstrated to be higher in the pediatric population, higher or more frequent dosing based on body weight may be needed. [See Clinical Pharmacology (12.3)]
Safety and efficacy studies have been performed in 146 pediatric patients <18 years of age. Ninety (including all 59 PUPs) of these subjects (62%) were <6 years of age, 32 (22%) were 6 to <12 years of age, and 24 (16%) were adolescents (12 to <18 years of age). Subjects during routine prophylaxis and treatment of bleeds received Novoeight at the dose levels described in Tables 1 and 3. A total of 1290 bleeds in 127 subjects were treated with Novoeight. The majority of the bleeds 1162 (90%) were of mild/moderate severity. Of these 1290 bleeds, 1140 (88%) were rated excellent or good in their response to treatment with Novoeight and in 17 (1%) the response to treatment was unknown. A total of 1100 (85%) of the bleeds were resolved with one or two injections of Novoeight. Routine prophylactic treatment has been shown to reduce joint bleeding. [See Clinical Studies (14)]
8.5 Geriatric Use
Clinical studies of Novoeight did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
8.6 Obesity
In the extension trial, in six adult patients with body mass index (BMI) ≥ 30 kg/m2, the AUC was higher and clearance lower than in patients with BMI < 30 kg/m2. There is insufficient data to recommend specific dose adjustments for patients with BMI ≥ 30 kg/m2. Adjust dose as necessary and per prescriber’s discretion for patients with BMI ≥ 30 kg/m2. [See Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
Novoeight is formulated as a sterile, non-pyrogenic, lyophilized powder for intravenous injection after reconstitution with the diluent (0.9% sodium chloride). Novoeight is available in single-dose vials that contain nominally 250, 500, 1000, 1500, 2000 or 3000 international units (IU) per vial. When reconstituted with the appropriate volume of diluent, the product contains the following components per mL: 18 mg sodium chloride, 1.5 mg L-histidine, 3 mg sucrose, 0.1 mg polysorbate 80, 0.055 mg L-methionine and 0.25 mg calcium chloride dihydrate. The product contains no preservative. Each vial of Novoeight is labeled with the actual rFVIII activity expressed in IU determined by the one-stage clotting assay, using a reference material calibrated against a World Health Organization (WHO) International Standard for FVIII Concentrates. One IU, as defined by the WHO standard for human FVIII, is approximately equal to the level of FVIII activity in 1 mL of fresh pooled human plasma. The specific activity of Novoeight is approximately 8340 IU per milligram of protein.
The active ingredient in Novoeight is a recombinant (r) analogue of human coagulation factor VIII (FVIII) with a molecular mass of 166 kDa, calculated excluding post-translational modifications. The rFVIII molecule in Novoeight is a glycoprotein containing a heavy chain and a light chain, with 21 of the 908 amino acids of the B-domain of endogenous FVIII connected to the C-terminus of the heavy chain. Once activated, the resulting rFVIIIa has a comparable structure to the endogenous FVIIIa.
Novoeight is synthesized by a genetically engineered Chinese hamster ovary (CHO) cell line which secretes rFVIII into the cell culture medium. The rFVIII protein is purified using a series of chromatography steps, one of which is the use of an immunoaffinity column in which a monoclonal antibody, produced in CHO cells and directed against FVIII, is employed to selectively isolate the rFVIII from the medium. The production process includes two dedicated viral clearance steps - a detergent treatment step for inactivation and a 20-nm filtration step for removal of viruses. No additives of human or animal origin are used in the cell culture, purification and formulation of Novoeight.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Novoeight temporarily replaces the missing clotting factor VIII that is needed for effective hemostasis.
12.2 Pharmacodynamics
The activated partial thromboplastin time (aPTT) is prolonged in patients with hemophilia A. Determination of aPTT is a conventional in vitro assay for the biological activity of FVIII. Treatment with Novoeight normalizes the aPTT over the effective dosing period.
12.3 Pharmacokinetics
All pharmacokinetic studies with Novoeight were conducted in previously treated patients with severe hemophilia A (factor VIII ≤ 1%). Analysis of plasma samples was conducted using both the one-stage clotting assay and the chromogenic assay.
In a multi-center, multi-national, open-label, single dose pharmacokinetic study, 23 patients with severe hemophilia A received 50 international units/kg of Novoeight intravenously. Two patients were below the age of 18 years (13 and 17 years). The pharmacokinetic parameters for 20 patients who completed the study are summarized in Table 5.
Table 5: Pharmacokinetics of Novoeight in 20 adult and adolescent patients with hemophilia Aa
Parameters
Clotting Assay
Chromogenic Assay
Mean (SD)
Mean (SD)
Incremental Recovery (IU/mL)/(IU/kg)
0.020 (0.002)
0.028 (0.006)
AUC (IU*h/mL)
14.2 (3.8)
18.7 (5.1)
CL (mL/h/kg)
3.74 (0.95)
2.87 (0.80)
t½ (h)
10.8 (4.9)
12.0 ( 9.3)
Vss (mL/kg)
53.4 (10.9)
44.3 (28.2)
Cmax (IU/mL)
1.07 (0.16)
1.54 (0.29)
MRT (h)
15.4 (6.4)
16.4 (10.1)
aDose: 50 IU/kg turoctocog alfa (single i.v. dose)
In a single dose PK assessment in adult patients with BMI ≥ 30 kg/m2 in the extension trial [See Clinical Studies (14)], the AUC was 59% higher and clearance was 33% lower in 6 subjects with BMI ≥ 30 kg/m2 compared to subjects with normal BMI, see Table 6.
Table 6: Pharmacokinetics of Novoeight in 6 adult patients with BMI ≥ 30 kg/m2a
Parameters Clotting Assay Chromogenic Assay Mean (SD) Mean (SD) BMI (kg/m2)
33.35 (2.367)
Range 30.5 – 37.2
Incremental Recovery (IU/mL)/(IU/kg)
0.024 (0.01)
0.035 (0.01)
AUC (IU*h/mL)
22.64 (5.74)
31.02 (9.78)
CL (mL/h/kg)
2.49 (0.77)
1.94 (0.95)
t½ (h)
12.80 (2.99)
12.40 (3.16)
Vss (mL/kg)
39.67 (10.03)
29.79 (7.87)
Cmax (IU/mL)
1.49 (0.36)
2.03 (0.51)
MRT (h)
16.84 (4.78)
16.58 (4.26)
aDose: 50 IU/kg turoctocog alfa (single i.v. dose)
In a separate pharmacokinetic study, 28 pediatric patients with severe hemophilia A (14 patients were below 6 years of age and 14 patients were between 6 to <12 years of age) received a single dose of 50 international units/kg Novoeight. The pharmacokinetic parameters of Novoeight are summarized in Table 7 for both age groups.
Table 7: Pharmacokinetics of Novoeight in 28 pediatric patients with hemophilia A
Parameters
Clotting Assay
Chromogenic Assay
0 to <6 years
6 to <12 years
0 to <6 years
6 to <12 years
Mean (SD)
Mean (SD)
Incremental Recovery (IU/mL)/(IU/kg)
0.018 (0.007)
0.020 (0.004)
0.022 (0.006)
0.025 (0.006)
AUC (IU*h/mL)
9.9 (4.1)
11.1 (3.7)
12.2 (4.4)
14.4 (3.5)
CL (mL/h/kg)
6.26 (3.73)
5.02 (1.67)
4.60 (1.75)
3.70 (1.00)
t½ (h)
7.7 (1.8)
8.0 (1.9)
10.0 (1.7)
9.4 (1.5)
Vss (mL/kg)
57.3 (26.8)
46.8 (10.6)
55.8 (23.7)
41.2 (6.0)
Cmax (IU/mL)
1.00 (0.58)
1.07 (0.35)
1.12 (0.31)
1.25 (0.27)
MRT (h)
9.7 (2.5)
9.9 (2.6)
12.1 (1.9)
11.6 (2.3)
The pharmacokinetic parameters were comparable between younger (0 to < 6 years) and older (6 to < 12 years) children. The mean clearance of Novoeight in younger and older children was 67% and 34% higher (based on per kg body weight) than in adults (3.74 mL/h/kg) when using the clotting assay, and 60% and 29% higher than in adults (2.87 mL/h/kg) when using the chromogenic assay. The mean half-life of Novoeight in younger and older children was 29% and 26% shorter than in adults (10.8 hours) when using the clotting assay, and 16% and 21% shorter than in adults (12 hours) when using the chromogenic assay.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of Novoeight, or studies to determine the effects of Novoeight on genotoxicity or fertility have not been performed. An assessment of the carcinogenic potential of Novoeight was completed, and no carcinogenic risk from product use has been identified.
-
14 CLINICAL STUDIES
Four multi-center, open-label, non-controlled trials have been conducted to evaluate the safety and efficacy of Novoeight in the on-demand treatment and control of breakthrough bleeds, routine prophylaxis and perioperative management in patients with hemophilia A. Three of these trials were performed in PTPs (two trials and one extension trial) and the fourth in PUPs. The analysis included 297 exposed subjects: 175 previously treated adolescents or adult subjects from the age of 12 years (≥150 exposure days), 63 previously treated pediatric subjects below the age of 12 years (≥50 exposure days) and 59 PUPs below 6 years of age. Immunocompetent patients with severe hemophilia A (factor VIII activity ≤1%) and no history of FVIII inhibitors were eligible for the trials. Subjects during routine prophylaxis and treatment of bleeds received Novoeight at the dose levels described in Tables 1 and 3. Breakthrough bleeds were treated at the investigator’s discretion aiming for a FVIII activity level above 0.5 IU/mL. Treatment during surgery was at the investigator’s discretion aiming for a FVIII trough activity level above 0.5 IU/mL.
On-demand Treatment and Control of Bleeding Episodes
A total of 3153 bleeds in 260 subjects were treated with Novoeight. The majority of the bleeds (90%) were of mild/moderate severity, 54% of the bleeds were spontaneous and 67% of the bleeds were localized in joints.
An overall assessment of efficacy was performed by the subject (for home treatment) or study site investigator (for treatment under medical supervision) using a four-point scale of excellent, good, moderate, or none. If the hemostatic response was rated as “excellent” or “good”, the treatment of the bleed was considered a success. If the hemostatic response was rated as “moderate or none” the treatment was considered a failure. Of these 3,153 bleeds, 2,809 (89%) were rated excellent or good in their response to treatment with Novoeight, 274 (9%) were rated as moderate, 25 (0.8%) were rated as having no response and for 45 (1%) the response to treatment was unknown. A total of 2,794 (89%) of the bleeds were resolved with one or two injections of Novoeight.
Of the 238 PTPs, 206 patients experienced 2,793 bleeds of which 2,492 (89%) were rated excellent or good in their response to treatment with Novoeight, 244 (9%) were moderate, 23 (0.8%) were rated as having no response, and for 34 (1%) the response to treatment was unknown. Of the 2,793 reported bleeds observed in 206 of the patients, 2,504 (90%) of the bleeds were resolved with 1–2 injections of Novoeight. The majority of the bleeds were of mild/moderate severity.
Of the 59 PUPs, 54 patients experienced 360 bleeds of which 317 (88%) were rated excellent or good in their response to treatment with Novoeight, 30 (8%) were moderate, 2 (0.6%) were rated as having no response, and for 11 (3%) the response to treatment was unknown. Of the 360 reported bleeds observed in 54 of the patients, 290 (81%) of the bleeds were resolved with 1–2 injections of Novoeight. The majority of the bleeds were of mild/moderate severity and the most frequent bleeds were subcutaneous.
Routine Prophylaxis
In the two trials, one trial including 150 adult/adolescent subjects (6 months duration) and the other trial including 63 pediatric subjects (4 months duration) received Novoeight for routine prophylaxis (Table 8). These previously treated patients received prophylaxis treatment every other day or three times weekly at the dose levels described in Table 3.
Table 8: Annualized Bleeding Rate (ABRa) for previously treated patients from the two trials
Small children
0 - <6 years
Older children
6 - <12 years
Adolescents
12 - <18 years
Adults
≥18 years
Total
Nb
31
32
24
126
213
Median (IQR)
2.97 (6.30)
3.65 (8.93)
3.98 (6.21)
3.70 (9.02)
3.67 (8.70)
Mean (95%CI)
4.77 (3.07; 7.41)
5.93 (3.81; 9.22)
5.48 (3.29; 9.14)
6.69 (5.36; 8.36)
6.24 (5.25; 7.41)
a: The ABRs were estimated using a Poisson model allowing for overdispersion.
b: Patients dosed every other day or three times weekly
Abbreviations: N: number of patients; IQR: interquartile range defined as the difference between the 75th percentile and the 25th percentile; CI: confidence interval.
One hundred and eighty-eight (188) subjects from the two trials above continued into the extension trial (up to 6 years duration) (Table 9). Additionally, 18 subjects (7 subjects from an on-demand sub-trial and 11 subjects from a pharmacokinetic trial) were included in the extension trial. These previously treated patients received prophylaxis treatment every other day or three times weekly at the dose levels described in Table 3.
Table 9: Annualized Bleeding Rate (ABRa) for previously treated patients from the extension trial
Small children
0 - <6 years
Older children
6 - <12 years
Adolescents
12 - <18 years
Adults
≥18 years
Total
Nb
27
28
23
128
206
Median (IQR)
1.08 (2.83)
1.57 (3.82)
1.57 (2.34)
1.38 (2.96)
1.39 (2.94)
Mean (95%CI)
1.87 (1.14; 3.09)
2.90 (2.01; 4.17)
1.93 (1.33; 2.82)
2.61 (2.08; 3.28)
2.45 (2.07; 2.90)
a: The ABRs were estimated using a Poisson model allowing for overdispersion.
b: Patients dosed every other day or three times weekly
Abbreviations: N: number of patients; IQR: interquartile range defined as the difference between the 75th percentile and the 25th percentile; CI: confidence interval.
In the trial with previously untreated patients, 56 subjects below 6 years of age received Novoeight for routine prophylaxis. The median annualized bleeding rate in the previously untreated patients was 2.9 (IQR 5.4) and the mean (95%CI) was 4.4 (3.3; 5.8).
Perioperative Management
A total of 30 surgeries were performed in 25 previously treated subjects between 8 and 58 years of age, of which 26 were major surgeries (20 orthopaedic, 5 non-orthopaedic and a circumcision), and 4 were minor (2 dental, 1 circumcision and 1 insertion of port-a-cath).
The investigator’s ratings of intra- and post-operative quality of hemostasis for these subjects were “excellent” or “good” for all cases.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- •
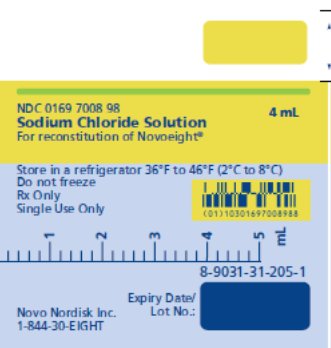
- Novoeight is supplied in packages comprised of a single-dose vial containing nominally 250, 500, 1000, 1500, 2000, or 3000 international units (IU) of FVIII potency, a MixPro® pre-filled diluent syringe containing 0.9% sodium chloride solution, and sterile vial adapter with 25 micrometer filter, which serves as a needleless reconstitution device.
- •
- The actual amount of FVIII potency in IU is stated on each carton and vial.
Presentation (Nominal Product Strength)
Carton NDC Number
Components
250 International Units
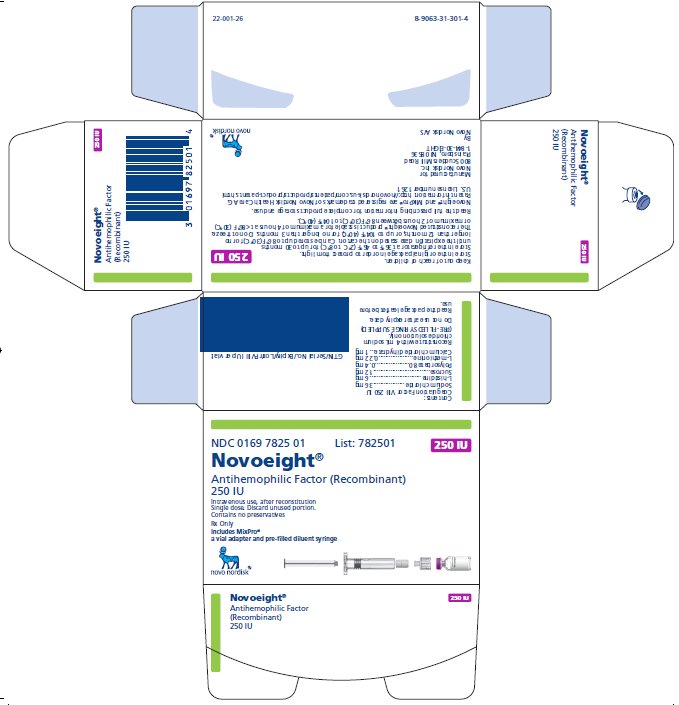
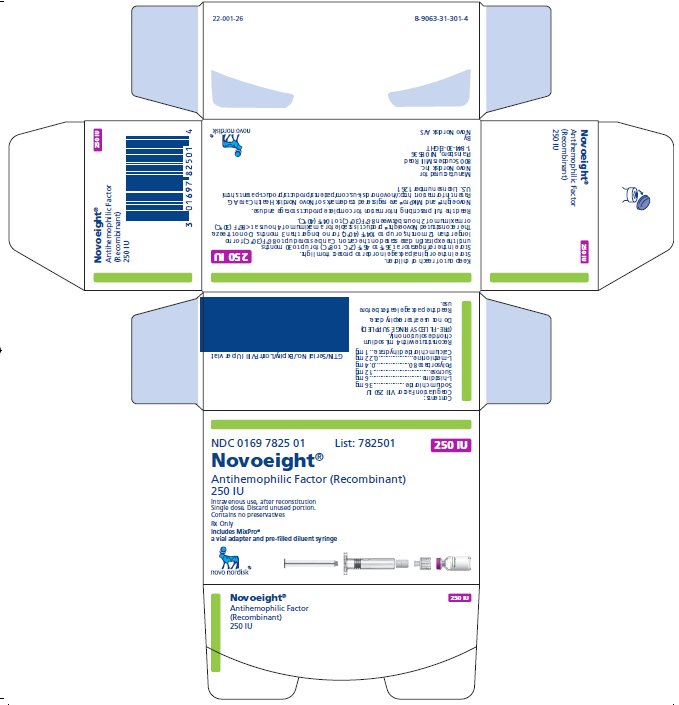
NDC 0169 7825 01
- •
- Novoeight in single-dose vial [NDC 0169-7829-11]
- •
- Pre-filled sodium chloride diluent in syringe, 4 mL [NDC 0169-7008-98]
- •
- Vial adapter
500 International Units
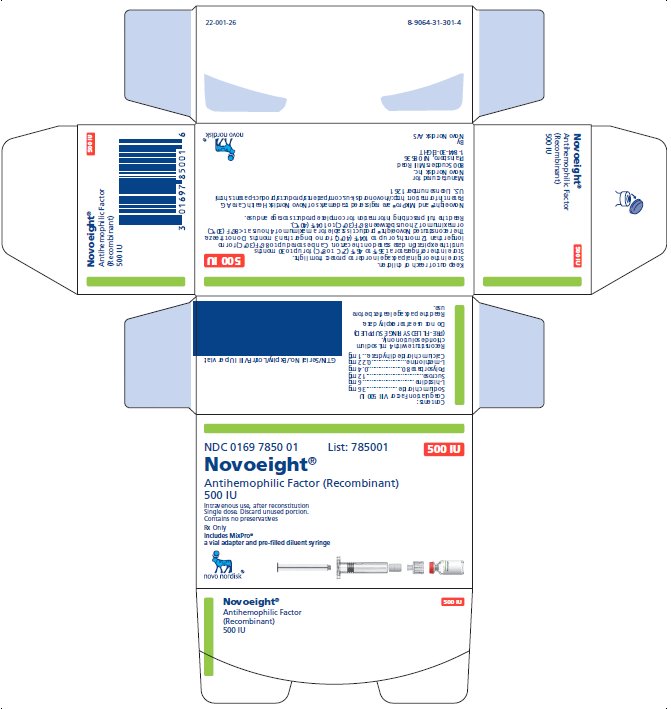
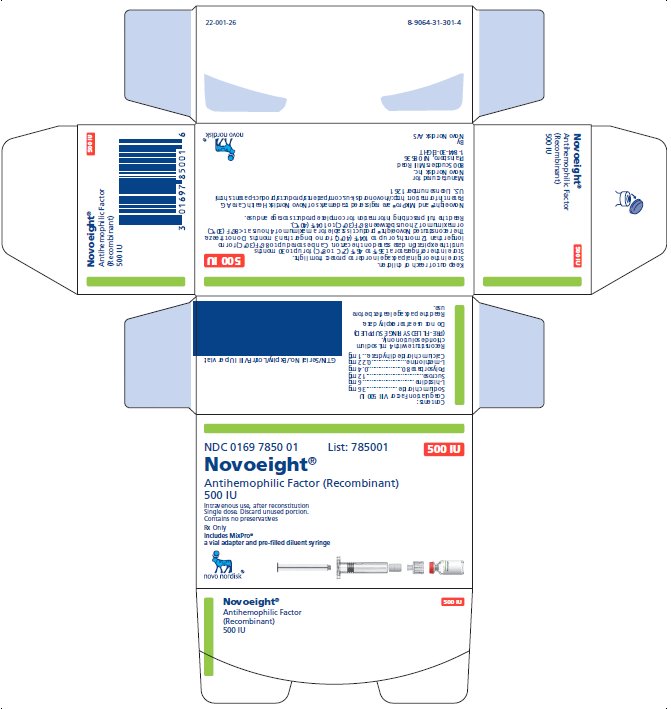
NDC 0169 7850 01
- •
- Novoeight in single-dose vial [NDC 0169-7851-11]
- •
- Pre-filled sodium chloride diluent in syringe, 4 mL [NDC 0169-7008-98]
- •
- Vial adapter
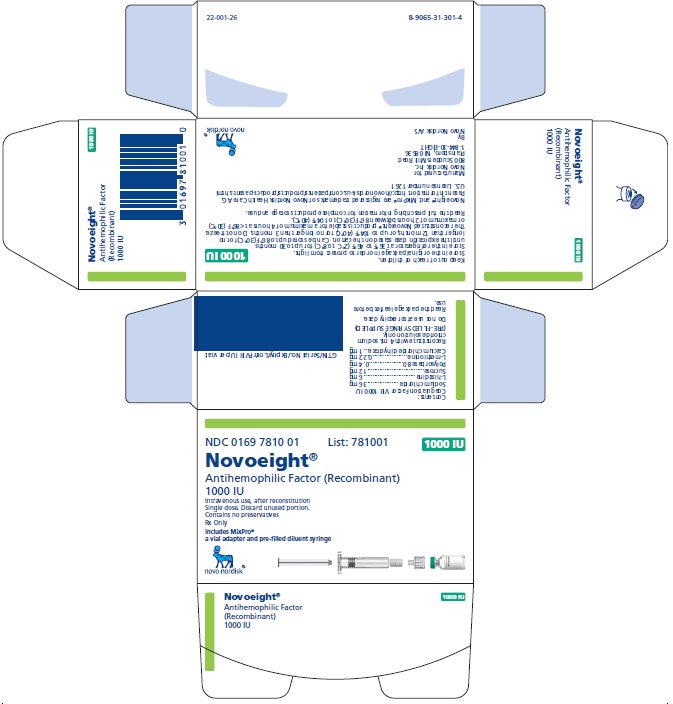
1000 International Units
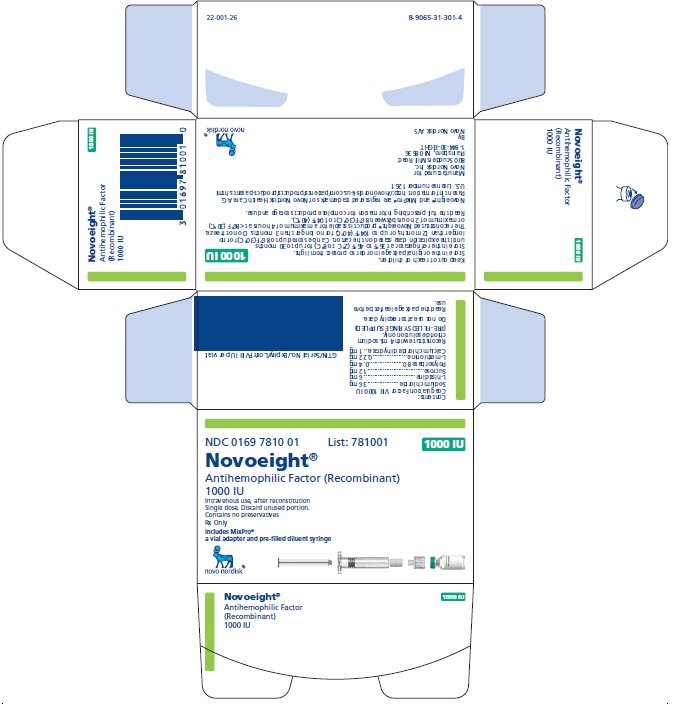
NDC 0169 7810 01
- •
- Novoeight in single-dose vial [NDC 0169-7811-11]
- •
- Pre-filled sodium chloride diluent in syringe, 4 mL [NDC 0169-7008-98]
- •
- Vial adapter
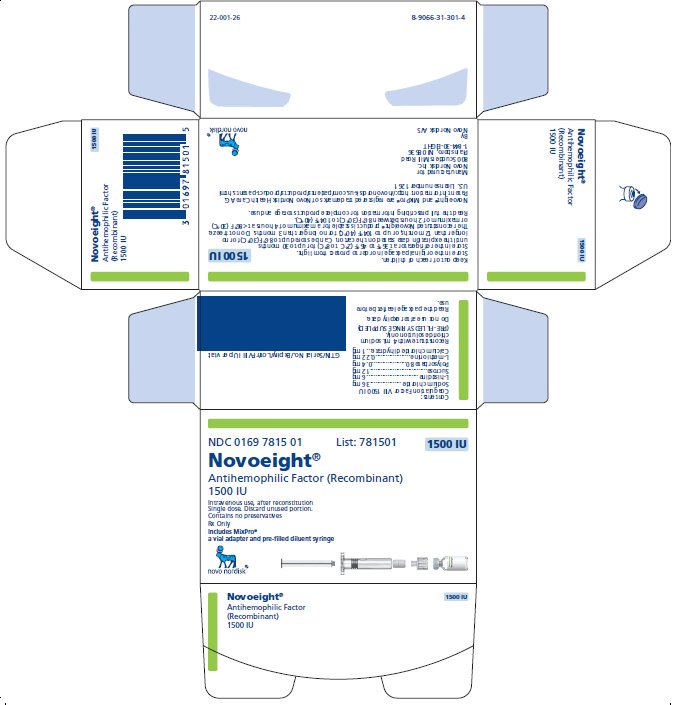
1500 International Units
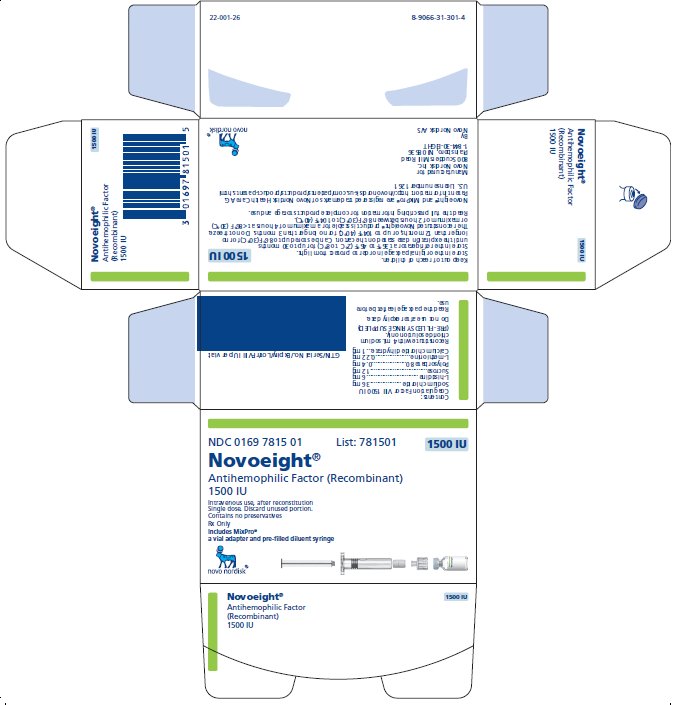
NDC 0169 7815 01
- •
- Novoeight in single-dose vial [NDC 0169-7855-11]
- •
- Pre-filled sodium chloride diluent in syringe, 4 mL [NDC 0169-7008-98]
- •
- Vial adapter
2000 International Units
NDC 0169 7820 01
- •
- Novoeight in single-dose vial [NDC 0169-7821-11]
- •
- Pre-filled sodium chloride diluent in syringe, 4 mL [NDC 0169-7008-98]
- •
- Vial adapter
3000 International Units
NDC 0169 7830 01
- •
- Novoeight in single-dose vial [NDC 0169-7831-11]
- •
- Pre-filled sodium chloride diluent in syringe, 4 mL [NDC 0169-7008-98]
- •
- Vial adapter
- •
- The Novoeight vials are made of glass, closed with a chlorobutyl rubber stopper not made with natural rubber latex, and sealed with an aluminum cap.
- •
- The pre-filled diluent syringes are made of glass, with a siliconised bromobutyl rubber plunger not made with natural rubber latex.
- •
- The closed vials and pre-filled diluent syringes are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Storage and Handling
- •
- Store Novoeight in the original package in order to protect from light.
- •
- Store Novoeight under refrigeration at a temperature of 36°F to 46°F (2°C to 8°C) for up to 30 months from the date of manufacture until the expiration date stated on the carton. During the 30-month shelf life, Novoeight may be kept at room temperature:
- •
- up to 86°F (≤30°C) for no longer than 12 months
- or
- •
- up to 104°F(≤40°C) for no longer than 3 months
- •
- Clearly record the date when the product was removed from the refrigerator in the space provided on the outer carton. Do not return the product to the refrigerator. Do not freeze Novoeight.
- •
- Use Novoeight within 4 hours after reconstitution when stored at <86°F (30°C) or within 2 hours when stored between 86°F (30°C) to 104°F (40°C). Store the reconstituted product in the vial.
- •
- Discard any unused reconstituted product.
-
17 PATIENT COUNSELING INFORMATION
- •
- Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Allergic-type hypersensitivity reactions or anaphylaxis are possible with use of Novoeight. Inform patients of the early signs of hypersensitivity reactions including rash, hives, itching, facial swelling, tightness of the chest and wheezing. Advise patients to discontinue use of Novoeight immediately and contact their physician, and go to the emergency department if these symptoms occur.
- •
- Advise patients to contact their physician or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
- •
- Advise patients to consult with their healthcare provider prior to traveling. While traveling, patients should be advised to bring an adequate supply of Novoeight based on their current treatment regimen.
Version: 8
License Number: 1261
Novoeight® and MixPro® are registered trademarks of Novo Nordisk Health Care AG.
Patent Information: http://novonordisk-us.com/patients/products/product-patents.html
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, Invision-Plus® Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector® is a registered trademark of Vygon.
© 2020 Novo Nordisk
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-844-30-EIGHT
Manufactured by:
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsvaerd
-
FDA approved Patient Labeling
Patient Product Information
Novoeight® (NŌ-vō-eyt)
Antihemophilic Factor (Recombinant)
Read the Patient Product Information and the Instructions For Use that come with Novoeight before you start taking this medicine and each time you get a refill. There may be new information.
This Patient Product Information does not take the place of talking with your healthcare provider about your medical condition or treatment. If you have questions about Novoeight after reading this information, ask your healthcare provider.
What is the most important information I need to know about Novoeight?
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing Novoeight so that your treatment will work best for you.
What is Novoeight?
Novoeight is an injectable medicine used to replace clotting factor VIII that is missing in patients with hemophilia A. Hemophilia A is an inherited bleeding disorder that prevents blood from clotting normally.
Novoeight is used to control and prevent bleeding in people with hemophilia A.
Your healthcare provider may give you Novoeight when you have surgery.
Novoeight is not used to treat von Willebrand Disease.
Who should not use Novoeight?
You should not use Novoeight if you
- •
- are allergic to factor VIII or any of the other ingredients of Novoeight
- •
- if you are allergic to hamster proteins
Tell your healthcare provider if you are pregnant or nursing because Novoeight might not be right for you.
What should I tell my healthcare provider before I use Novoeight?
You should tell your healthcare provider if you
- •
- Have or have had any medical conditions.
- •
- Take any medicines, including non-prescription medicines and dietary supplements.
- •
- Are nursing.
- •
- Are pregnant or planning to become pregnant.
- •
- Have been told that you have inhibitors to factor VIII.
How should I use Novoeight?
Treatment with Novoeight should be started by a healthcare provider who is experienced in the care of patients with hemophilia A.
Novoeight is given as an injection into the vein.
You may infuse Novoeight at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your hemophilia treatment center or healthcare provider. Many people with hemophilia A learn to infuse the medicine by themselves or with the help of a family member.
Your healthcare provider will tell you how much Novoeight to use based on your weight, the severity of your hemophilia A, and where you are bleeding.
You may need to have blood tests done after getting Novoeight to be sure that your blood level of factor VIII is high enough to clot your blood. This is particularly important if you are having major surgery.
Your healthcare provider will calculate your dose of Novoeight (in international units, IU) depending on your condition and body weight.
Call your healthcare provider right away if your bleeding does not stop after taking Novoeight.
Development of factor VIII inhibitors
Your body can also make antibodies called “inhibitors” against Novoeight, which may stop Novoeight from working properly.
If your bleeding is not adequately controlled, it could be due to the development of factor VIII inhibitors. This should be checked by your healthcare provider. You might need a higher dose of Novoeight or even a different product to control bleeding. Do not increase the total dose of Novoeight to control your bleeding without consulting your healthcare provider.
Use in children
Novoeight can be used in children. Your healthcare provider will decide the dose of Novoeight you will receive.
If you forget to use Novoeight
Do not inject a double dose to make up for a forgotten dose. Proceed with the next injections as scheduled and continue as advised by your healthcare provider.
If you stop using Novoeight
If you stop using Novoeight you are not protected against bleeding. Do not stop using Novoeight without consulting your healthcare provider.
If you have any further questions on the use of this product, ask your healthcare provider.
What if I take too much Novoeight?
Always take Novoeight exactly as your healthcare provider has told you. You should check with your healthcare provider if you are not sure. If you inject more Novoeight than recommended, tell your healthcare provider as soon as possible.
What are the possible side effects of Novoeight?
Common Side Effects Include:
- •
- Inhibitors in patients who were not previously treated with Factor VIII products
- •
- Swelling or itching at the location of injection
- •
- Fever
Other Possible Side Effects:
You could have an allergic reaction to coagulation factor VIII products. Call your healthcare provider right away and stop treatment if you get any of the following signs of an allergic reaction:
- •
- rashes including hives
- •
- difficulty breathing, shortness of breath or wheezing
- •
- tightness of the chest or throat, difficulty swallowing
- •
- swelling of the lips and tongue
- •
- light-headedness, dizziness or loss of consciousness
- •
- pale and cold skin, fast heart beat which may be signs of low blood pressure
- •
- red or swollen face or hands
These are not all of the possible side effects from Novoeight. Ask your healthcare provider for more information. You are encouraged to report side effects to FDA at 1-800-FDA-1088.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
What are the Novoeight dosage strengths?
Novoeight comes in six different dosage strengths. The actual number of international units (IU) of factor VIII in the vial will be imprinted on the label and on the box. The six different strengths are as follows:
Dosage strength of approximately 250 IU per vial
Dosage strength of approximately 500 IU per vial
Dosage strength of approximately 1000 IU per vial
Dosage strength of approximately 1500 IU per vial
Dosage strength of approximately 2000 IU per vial
Dosage strength of approximately 3000 IU per vial
Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your doctor.
How should I store Novoeight?
Prior to Reconstitution:
Store in original package in order to protect from light. Do not freeze Novoeight.
Novoeight vials can be stored in the refrigerator (36°F to 46°F [2°C to 8°C]) for up to 30 months or up to the expiration date. During the 30 month shelf life, the product may be kept at room temperature up to 86°F (30°C) for no longer than 12 months, or up to 104°F (40°C) for no longer than 3 months.
If you choose to store Novoeight at room temperature:
- •
- Note the date that the product is removed from refrigeration on the box.
- •
- Do not return the product to the refrigerator.
- •
- Do not use after 12 months if stored up to 86°F (30°C) or after 3 months if stored up to 104°F (40°C) or the expiration date listed on the vial, whichever is earlier.
Do not use this medicine after the expiration date which is on the outer carton and the vial. The expiration date refers to the last day of that month.
After Reconstitution (mixing the dry powder in the vial with the diluent):
The reconstituted Novoeight should appear clear to slightly unclear without particles.
The reconstituted Novoeight should be used immediately.
If you cannot use the Novoeight immediately after it is mixed, it must be used within 4 hours when stored at <86ºF (30°C) or within 2 hours when stored between 86°F (30°C) to 104°F (40°C). Store the reconstituted product in the vial.
Keep this medicine out of the sight and out of reach of children.
What else should I know about Novoeight and hemophilia A?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use Novoeight for a condition for which it is not prescribed. Do not share Novoeight with other people, even if they have the same symptoms that you have.
For more information about Novoeight, please call Novo Nordisk at 1-844-30-EIGHT.
Revised: 07/2020
Novoeight® is a registered trademark of Novo Nordisk Health Care AG.
Patent Information: http://novonordisk-us.com/patients/products/product-patents.html
© 2020 Novo Nordisk
Manufactured by:
- Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about Novoeight® contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
- Principal Display Panel
- 500 IU Carton
- 1000 IU Carton
- 1500 IU Carton
- 2000 IU Carton
- 3000 IU Carton
- 250 IU Vial Label
- 500 IU Vial Label
- 1000 IU Vial Label
- 1500 IU Vial Label
- 2000 IU Vial Label
- 3000 IU Vial Label
- Syringe Label
-
INGREDIENTS AND APPEARANCE
NOVOEIGHT
antihemophilic factor recombinant kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0169-7825 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7825-01 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 NOVOEIGHT
antihemophilic factor recombinant injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0169-7829 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 62.5 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 18 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 3 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.055 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7829-11 4 mL in 1 VIAL, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Part 2 of 2 SODIUM CHLORIDE SOLUTION
sodium chloride solution liquidProduct Information Item Code (Source) NDC:0169-7008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7008-98 4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 NOVOEIGHT
antihemophilic factor recombinant kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0169-7850 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7850-01 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 NOVOEIGHT
antihemophilic factor recombinant injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0169-7851 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 125 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 18 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 3 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.055 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7851-11 4 mL in 1 VIAL, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Part 2 of 2 SODIUM CHLORIDE SOLUTION
sodium chloride solution liquidProduct Information Item Code (Source) NDC:0169-7008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7008-98 4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 NOVOEIGHT
antihemophilic factor recombinant kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0169-7810 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7810-01 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 NOVOEIGHT
antihemophilic factor recombinant injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0169-7811 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 250 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 18 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 3 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.055 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7811-11 4 mL in 1 VIAL, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Part 2 of 2 SODIUM CHLORIDE SOLUTION
sodium chloride solution liquidProduct Information Item Code (Source) NDC:0169-7008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7008-98 4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 NOVOEIGHT
antihemophilic factor recombinant kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0169-7815 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7815-01 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 NOVOEIGHT
antihemophilic factor recombinant injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0169-7855 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 375 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 18 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 3 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.055 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7855-11 4 mL in 1 VIAL, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Part 2 of 2 SODIUM CHLORIDE SOLUTION
sodium chloride solution liquidProduct Information Item Code (Source) NDC:0169-7008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7008-98 4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 NOVOEIGHT
antihemophilic factor recombinant kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0169-7820 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7820-01 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 NOVOEIGHT
antihemophilic factor recombinant injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0169-7821 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 18 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 3 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.055 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7821-11 4 mL in 1 VIAL, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Part 2 of 2 SODIUM CHLORIDE SOLUTION
sodium chloride solution liquidProduct Information Item Code (Source) NDC:0169-7008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7008-98 4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 NOVOEIGHT
antihemophilic factor recombinant kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0169-7830 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7830-01 1 in 1 KIT; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 4 mL Part 2 1 SYRINGE, GLASS 4 mL Part 1 of 2 NOVOEIGHT
antihemophilic factor recombinant injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0169-7831 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 750 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 18 mg in 1 mL HISTIDINE (UNII: 4QD397987E) 1.5 mg in 1 mL SUCROSE (UNII: C151H8M554) 3 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.055 mg in 1 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7831-11 4 mL in 1 VIAL, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Part 2 of 2 SODIUM CHLORIDE SOLUTION
sodium chloride solution liquidProduct Information Item Code (Source) NDC:0169-7008 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0169-7008-98 4 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125466 04/01/2015 Labeler - Novo Nordisk (622920320)