Label: DIPYRIDAMOLE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-2996-0 - Packager: Carilion Materials Management
- This is a repackaged label.
- Source NDC Code(s): 0054-0434
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated February 15, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Dipyridamole Tablets USP are a platelet inhibitor chemically described as 2,2',2'',2'''-[(4,8-Dipiperidinopyrimido[5,4- ]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula: d
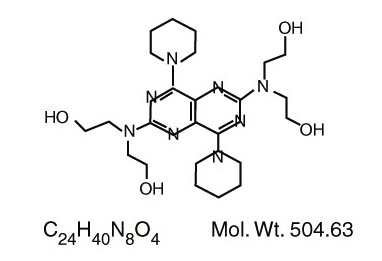
Dipyridamole is an odorless yellow crystalline powder, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and practically insoluble in water.
Dipyridamole Tablets USP for oral administration contain:
dipyridamole USP 25 mg, 50 mg and 75 mg, respectively. Active IngredientTABLETS 25 mg, 50 mg, and 75mg:
acacia, carnauba wax, corn starch, edible white ink, lactose monohydrate, magnesium stearate, D&C yellow #10 aluminum lake, D&C red #30, helendon aluminum pink lake, sodium benzoate, methylparaben, propylparaben, polyethylene glycol, povidone, sucrose, talc, titanium dioxide, and white wax. Inactive IngredientsTABLETS 25 mg, 50 mg, and75 mg:
-
CLINICAL PHARMACOLOGY
It is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic complications occurring in connection with prosthetic heart valve replacement.
Dipyridamole tablets have been found to lengthen abnormally shortened platelet survival time in a dose-dependent manner.
In three randomized controlled clinical trials involving 854 patients who had undergone surgical placement of a prosthetic heart valve, dipyridamole tablets, in combination with warfarin, decreased the incidence of postoperative thromboembolic events by 62 to 91% compared to warfarin treatment alone. The incidence of thromboembolic events in patients receiving the combination of dipyridamole tablets and warfarin ranged from 1.2 to 1.8%. In three additional studies involving 392 patients taking dipyridamole tablets and coumarin-like anticoagulants, the incidence of thromboembolic events ranged from 2.3 to 6.9%.
In these trials, the coumarin anticoagulant was begun between 24 hours and 4 days postoperatively, and the dipyridamole tablets USP were begun between 24 hours and 10 days postoperatively. The length of follow-up in these trials varied from 1 to 2 years.
Dipyridamole tablets do not influence prothrombin time or activity measurements when administered with warfarin.
Mechanism of Action
Dipyridamole inhibits the uptake of adenosine into platelets, endothelial cells and erythrocytes and ; the inhibition occurs in a dose-dependent manner at therapeutic concentrations (0.5-1.9 μg/mL). This inhibition results in an increase in local concentrations of adenosine which acts on the platelet A2-receptor thereby stimulating platelet adenylate cyclase and increasing platelet cyclic-3',5'-adenosine monophosphate (cAMP) levels. Via this mechanism, platelet aggregation is inhibited in response to various stimuli such as platelet activating factor (PAF), collagen and adenosine diphosphate (ADP). in vitroin vivo
Dipyridamole inhibits phosphodiesterase (PDE) in various tissues. While the inhibition of cAMPPDE is weak, therapeutic levels of dipyridamole inhibit cyclic-3',5'-guanosine monophosphate-PDE (cGMP-PDE), thereby augmenting the increase in cGMP produced by EDRF (endothelium-derived relaxing factor, now identified as nitric oxide).
Hemodynamics
In dogs intraduodenal doses of dipyridamole of 0.5 to 4.0 mg/kg produced dose-related decreases in systemic and coronary vascular resistance leading to decreases in systemic blood pressure and increases in coronary blood flow. Onset of action was in about 24 minutes and effects persisted for about 3 hours.
Similar effects were observed following IV dipyridamole in doses ranging from 0.025 to 2.0 mg/kg.
In man the same qualitative hemodynamic effects have been observed. However, acute intravenous administration of dipyridamole may worsen regional myocardial perfusion distal to partial occlusion of coronary arteries.
Pharmacokinetics and Metabolism
Following an oral dose of dipyridamole tablets, the average time to peak concentration is about 75 minutes. The decline in plasma concentration following a dose of dipyridamole tablets fits a two-compartment model. The alpha half-life (the initial decline following peak concentration) is approximately 40 minutes. The beta half-life (the terminal decline in plasma concentration) is approximately 10 hours. Dipyridamole is highly bound to plasma proteins. It is metabolized in the liver where it is conjugated as a glucuronide and excreted with the bile.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease (e.g., unstable angina or recently sustained myocardial infarction). Chest pain may be aggravated in patients with underlying coronary artery disease who are receiving dipyridamole. Coronary Artery Disease:
Elevations of hepatic enzymes and hepatic failure have been reported in association with dipyridamole administration. Hepatic Insufficiency:
Dipyridamole should be used with caution in patients with hypotension since it can produce peripheral vasodilation. Hypotension:
Drug Interactions
No pharmacokinetic drug-drug interaction studies were conducted with dipyridamole tablets USP. The following information was obtained from the literature.
Dipyridamole has been reported to increase the plasma levels and cardiovascular effects of adenosine. Adjustment of adenosine dosage may be necessary. Adenosine:
Dipyridamole may counteract the anticholinesterase effect of cholinesterase inhibitors, thereby potentially aggravating myasthenia gravis. Cholinesterase Inhibitors:
Carcinogenesis, Mutagenesis, Impairment of Fertility
In studies in which dipyridamole was administered in the feed to mice (up to 111 weeks in males and females) and rats (up to 128 weeks in males and up to 142 weeks in females), there was no evidence of drug-related carcinogenesis.
The highest dose administered in these studies (75 mg/kg/day) was, on a mg/m basis, about equivalent to the maximum recommended daily human oral dose (MRHD) in mice and about twice the MRHD in rats. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (about 12 times the MRHD on a mg/m basis). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg (more than 30 times the MRHD on a mg/m basis). 222
Pregnancy
Teratogenic Effect
Reproduction studies have been performed in mice, rabbits and rats at oral dipyridamole doses of up to 125 mg/kg, 40 mg/kg and 1000 mg/kg, respectively (about 11⁄2, 2 and 25 times the maximum recommended daily human oral dose, respectively, on a mg/m basis) and have revealed no evidence of harm to the fetus due to dipyridamole. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, dipyridamole tablets should be used during pregnancy only if clearly needed. Pregnancy Category B.2
-
ADVERSE REACTIONS
Adverse reactions at therapeutic doses are usually minimal and transient. On long-term use of dipyridamole tablets initial side effects usually disappear. The following reactions in Table 1 were reported in two heart valve replacement trials comparing dipyridamole tablets and war- farin therapy to either warfarin alone or warfarin and placebo:
Table 1 Adverse Reactions Reported in 2 Heart Valve Replacement Trials Adverse Reaction Dipyridamole Tablets/Warfarin Placebo/Warfarin Number of Patients 147 170 Dizziness 13.6% 8.2% Abdominal distress 6.1% 3.5% Headache 2.3% 0.0% Rash 2.3% 1.1% Other reactions from uncontrolled studies include diarrhea, vomiting, flushing and pruritus. In addition, angina pectoris has been reported rarely and there have been rare reports of liver dysfunction. On those uncommon occasions when adverse reactions have been persistent or intolerable, they have ceased on withdrawal of the medication.
When dipyridamole tablets USP were administered concomitantly with warfarin, bleeding was no greater in frequency or severity than that observed when warfarin was administered alone. In rare cases, increased bleeding during or after surgery has been observed.
In post-marketing reporting experience, there have been rare reports of hypersensitivity reac- tions (such as rash, urticaria, severe bronchospasm, and angioedema), larynx edema, fatigue, malaise, myalgia, arthritis, nausea, dyspepsia, paresthesia, hepatitis, thrombocytopenia, alo- pecia, cholelithiasis, hypotension, palpitation, and tachycardia.
-
OVERDOSAGE
In case of real or suspected overdose, seek medical attention or contact a Poison Control Center immediately. Careful medical management is essential. Based upon the known hemodynamic effects of dipyridamole, symptoms such as warm feeling, flushes, sweating, restlessness, feeling of weakness and dizziness may occur. A drop in blood pressure and tachycardia might also be observed.
Symptomatic treatment is recommended, possibly including a vasopressor drug. Gastric lavage should be considered. Administration of xanthine derivatives (e.g., aminophylline) may reverse the hemodynamic effects of dipyridamole overdose. Since dipyridamole is highly protein bound, dialysis is not likely to be of benefit.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Dipyridamole 25 MG TAB
-
INGREDIENTS AND APPEARANCE
DIPYRIDAMOLE
dipyridamole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68151-2996(NDC:0054-0434) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPYRIDAMOLE (UNII: 64ALC7F90C) (DIPYRIDAMOLE - UNII:64ALC7F90C) DIPYRIDAMOLE 25 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) SODIUM BENZOATE (UNII: OJ245FE5EU) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONES (UNII: FZ989GH94E) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WHITE WAX (UNII: 7G1J5DA97F) Product Characteristics Color ORANGE Score no score Shape ROUND Size 5mm Flavor Imprint Code 17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-2996-0 1 in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA012836 02/15/2012 Labeler - Carilion Materials Management (079239644) Registrant - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-2996)


