Label: DAYWEAR ADVANCED MULTI-PROTECTION ANTI-OXIDANT 24H-MOISTURE CREME BROAD SPECTRUM SPF15- avobenzone, homosalate, octisalate cream
- NDC Code(s): 11559-064-01
- Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
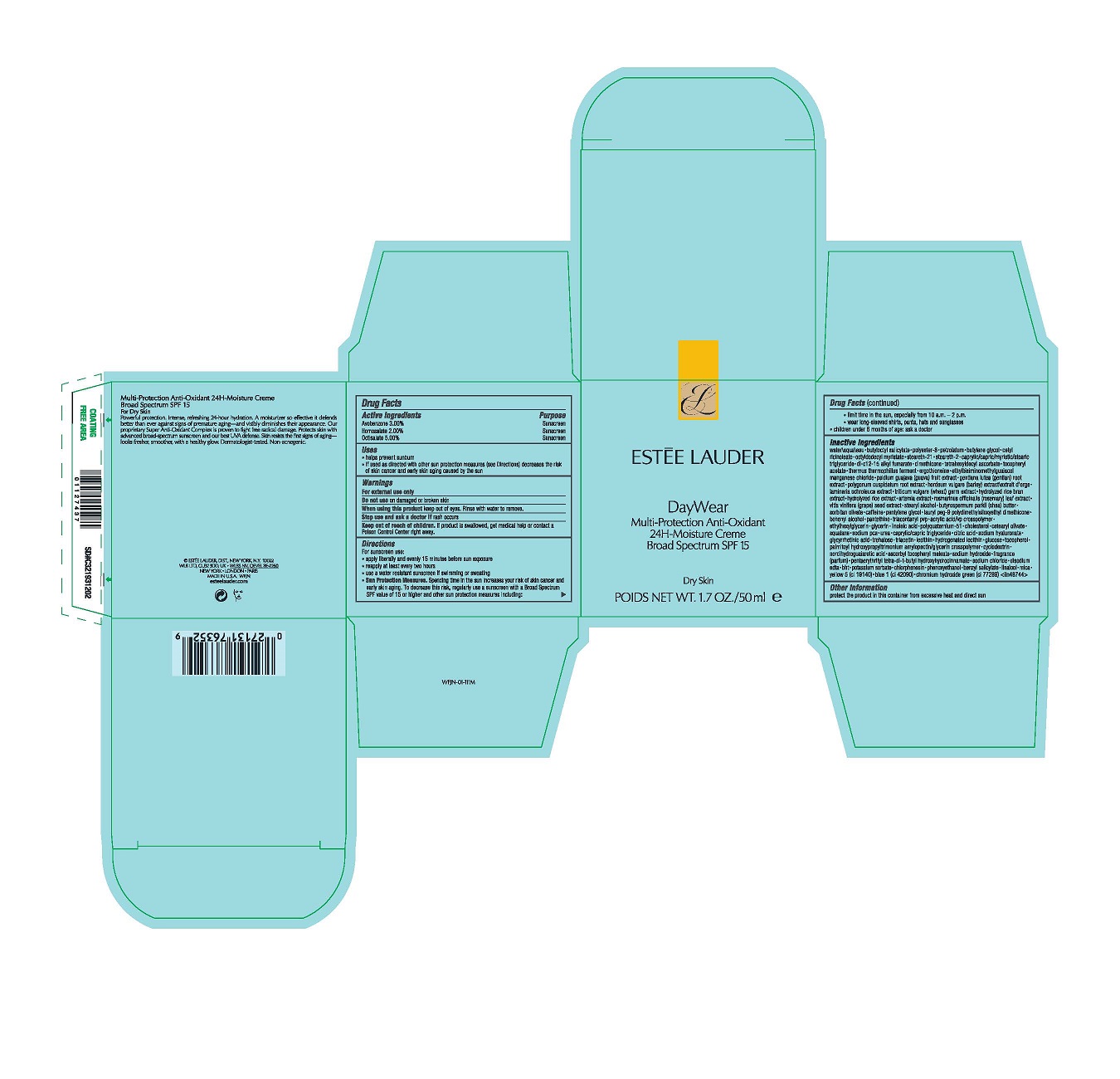
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
For sunscreen use:
apply liberally 15 minutes before sun exposure
reapply at least every two hours
use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m.–2 p.m.
wear long-sleeved shirts, pants, hats and sunglasses
children under 6 months of age: ask a doctor -
Inactive Ingredients
WATER\AQUA\EAU,BUTYLOCTYL SALICYLATE,POLYESTER-8,PETROLATUM,BUTYLENE GLYCOL,CETYL RICINOLEATE,OCTYLDODECYL MYRISTATE,STEARETH-21,STEARETH-2,CAPRYLIC/CAPRIC/MYRISTIC/STEARIC TRIGLYCERIDE,DI-C12-15 ALKYL FUMARATE,DIMETHICONE,TETRAHEXYLDECYL ASCORBATE,TOCOPHERYL ACETATE,THERMUS THERMOPHILLUS FERMENT,ERGOTHIONEINE,ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE,PSIDIUM GUAJAVA (GUAVA) FRUIT EXTRACT,GENTIANA LUTEA (GENTIAN) ROOT EXTRACT,POLYGONUM CUSPIDATUM ROOT EXTRACT,HORDEUM VULGARE (BARLEY) EXTRACT\EXTRAIT D'ORGE,LAMINARIA OCHROLEUCA EXTRACT,TRITICUM VULGARE (WHEAT) GERM EXTRACT,HYDROLYZED RICE BRAN EXTRACT,HYDROLYZED RICE EXTRACT,ARTEMIA EXTRACT,ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT,VITIS VINIFERA (GRAPE) SEED EXTRACT,STEARYL ALCOHOL,BUTYROSPERMUM PARKII (SHEA) BUTTER,SORBITAN OLIVATE,CAFFEINE,PENTYLENE GLYCOL,LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE,BEHENYL ALCOHOL,PANTETHINE,TRIACONTANYL PVP,ACRYLIC ACID/VP CROSSPOLYMER,ETHYLHEXYLGLYCERIN,GLYCERIN,LINOLEIC ACID,POLYQUATERNIUM-51,CHOLESTEROL,CETEARYL OLIVATE,SQUALANE,SODIUM PCA,UREA,CAPRYLIC/CAPRIC TRIGLYCERIDE,CITRIC ACID,SODIUM HYALURONATE,GLYCYRRHETINIC ACID,TREHALOSE,TRIACETIN,LECITHIN,HYDROGENATED LECITHIN,GLUCOSE,TOCOPHEROL,PALMITOYL HYDROXYPROPYLTRIMONIUM AMYLOPECTIN/GLYCERIN CROSSPOLYMER,CYCLODEXTRIN,NORDIHYDROGUAIARETIC ACID,ASCORBYL TOCOPHERYL MALEATE,SODIUM HYDROXIDE,FRAGRANCE (PARFUM),PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE,SODIUM CHLORIDE,DISODIUM EDTA,BHT,POTASSIUM SORBATE,CHLORPHENESIN,PHENOXYETHANOL,BENZYL SALICYLATE,LINALOOL,MICA,YELLOW 5 (CI 19140),BLUE 1 (CI 42090),CHROMIUM HYDROXIDE GREEN (CI 77289) <ILN48744>
- Other information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DAYWEAR ADVANCED MULTI-PROTECTION ANTI-OXIDANT 24H-MOISTURE CREME BROAD SPECTRUM SPF15
avobenzone, homosalate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 20 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength DI-C12-15 ALKYL FUMARATE (UNII: A1CB3Z898P) VITIS VINIFERA SEED (UNII: C34U15ICXA) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) CHLORPHENESIN (UNII: I670DAL4SZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) SORBITAN OLIVATE (UNII: MDL271E3GR) CETEARYL OLIVATE (UNII: 58B69Q84JO) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CAPRYLIC/CAPRIC/MYRISTIC/STEARIC TRIGLYCERIDE (UNII: WHF5JJR62Y) ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE (UNII: SM5YJ88LTU) LAMINARIA OCHROLEUCA (UNII: 4R2124HE76) TRIACONTANYL PVP (WP-660) (UNII: N0SS3Q238D) LINOLEIC ACID (UNII: 9KJL21T0QJ) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ENOXOLONE (UNII: P540XA09DR) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) LINALOOL, (+/-)- (UNII: D81QY6I88E) MICA (UNII: V8A1AW0880) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) STEARETH-21 (UNII: 53J3F32P58) DIMETHICONE (UNII: 92RU3N3Y1O) GENTIANA LUTEA ROOT (UNII: S72O3284MS) WHEAT GERM (UNII: YR3G369F5A) STEARETH-2 (UNII: V56DFE46J5) DOCOSANOL (UNII: 9G1OE216XY) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLODEXTRINS (UNII: 7E6SK9QDT8) ASCORBYL TOCOPHERYL MALEATE (UNII: D2G6259XR5) POLYGONUM CUSPIDATUM ROOT (UNII: 7TRV45YZF7) ROSEMARY (UNII: IJ67X351P9) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) TREHALOSE (UNII: B8WCK70T7I) MASOPROCOL (UNII: 7BO8G1BYQU) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BENZYL SALICYLATE (UNII: WAO5MNK9TU) CETYL RICINOLEATE (UNII: 1P677500YD) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) GUAVA (UNII: 74O70D6VG0) CAFFEINE (UNII: 3G6A5W338E) PENTYLENE GLYCOL (UNII: 50C1307PZG) PANTETHINE (UNII: 7K81IL792L) SQUALANE (UNII: GW89575KF9) TRIACETIN (UNII: XHX3C3X673) OCTYLDODECYL MYRISTATE (UNII: S013N99GR8) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) ERGOTHIONEINE (UNII: BDZ3DQM98W) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TOCOPHEROL (UNII: R0ZB2556P8) PALMITOYL HYDROXYPROPYLTRIMONIUM AMYLOPECTIN/GLYCERIN CROSSPOLYMER (UNII: 7F9WLW26M2) SODIUM HYDROXIDE (UNII: 55X04QC32I) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) PETROLATUM (UNII: 4T6H12BN9U) HORDEUM VULGARE WHOLE (UNII: 8JBE478M5Q) SHEA BUTTER (UNII: K49155WL9Y) CHOLESTEROL (UNII: 97C5T2UQ7J) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) UREA (UNII: 8W8T17847W) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-064-01 1 in 1 CARTON 11/13/2023 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/13/2023 Labeler - ESTEE LAUDER INC (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(11559-064) , label(11559-064) , pack(11559-064)

 Estee Lauder
Estee Lauder