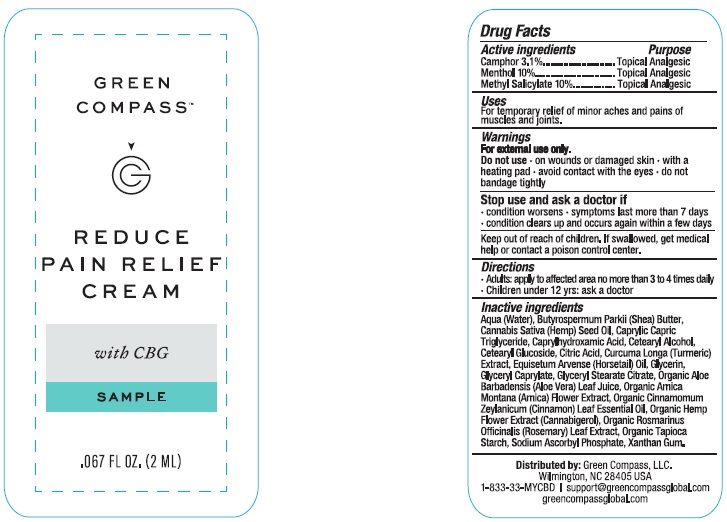
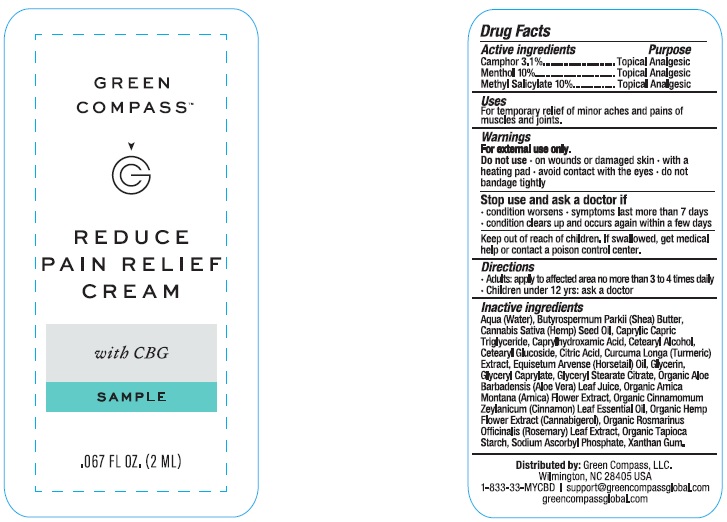
Label: GREEN COMPASS REDUCE PAIN RELIEF- camphor, menthol, methyl salicylate cream
- NDC Code(s): 73132-004-50
- Packager: Green Compass, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients
Aqua (water), butyrospermum parkii (shea) butter, cannabis sativa (hemp) seed oil, caprylhydroxamid acid, caprylic capric triglyceride, cetearyl alcohol, cetearyl glucoside, citric acid, curcuma longa (turmeric) extract, equisetum arvense (horsetail) oil, glycerin, glyceryl caprylate, glyceryl stearate citrate, hemp flower extract (cannabigerol), organic aloe barbadensis (aloe vera) leaf juice, organic arnica montana (arnica) flower extract, organic cinnamomum zeylanicum (cinnamon) leaf essential oil, organic rosmarinus officinalis (rosemary) leaf extract, organic tapioca starch, sodium ascorbyl phosphate, xanthan gum.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GREEN COMPASS REDUCE PAIN RELIEF
camphor, menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73132-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 3.1 g in 100 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) ARNICA MONTANA (UNII: O80TY208ZW) CANNABIGEROL (UNII: J1K406072N) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) CINNAMON LEAF OIL (UNII: S92U8SQ71V) EQUISETUM ARVENSE WHOLE (UNII: 73DM367W4P) TURMERIC (UNII: 856YO1Z64F) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HEMP (UNII: TD1MUT01Q7) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) SHEA BUTTER (UNII: K49155WL9Y) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) ALOE VERA LEAF (UNII: ZY81Z83H0X) ROSEMARY (UNII: IJ67X351P9) STARCH, TAPIOCA (UNII: 24SC3U704I) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73132-004-50 1 in 1 CARTON 11/01/2023 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 Labeler - Green Compass, Inc. (105963322)