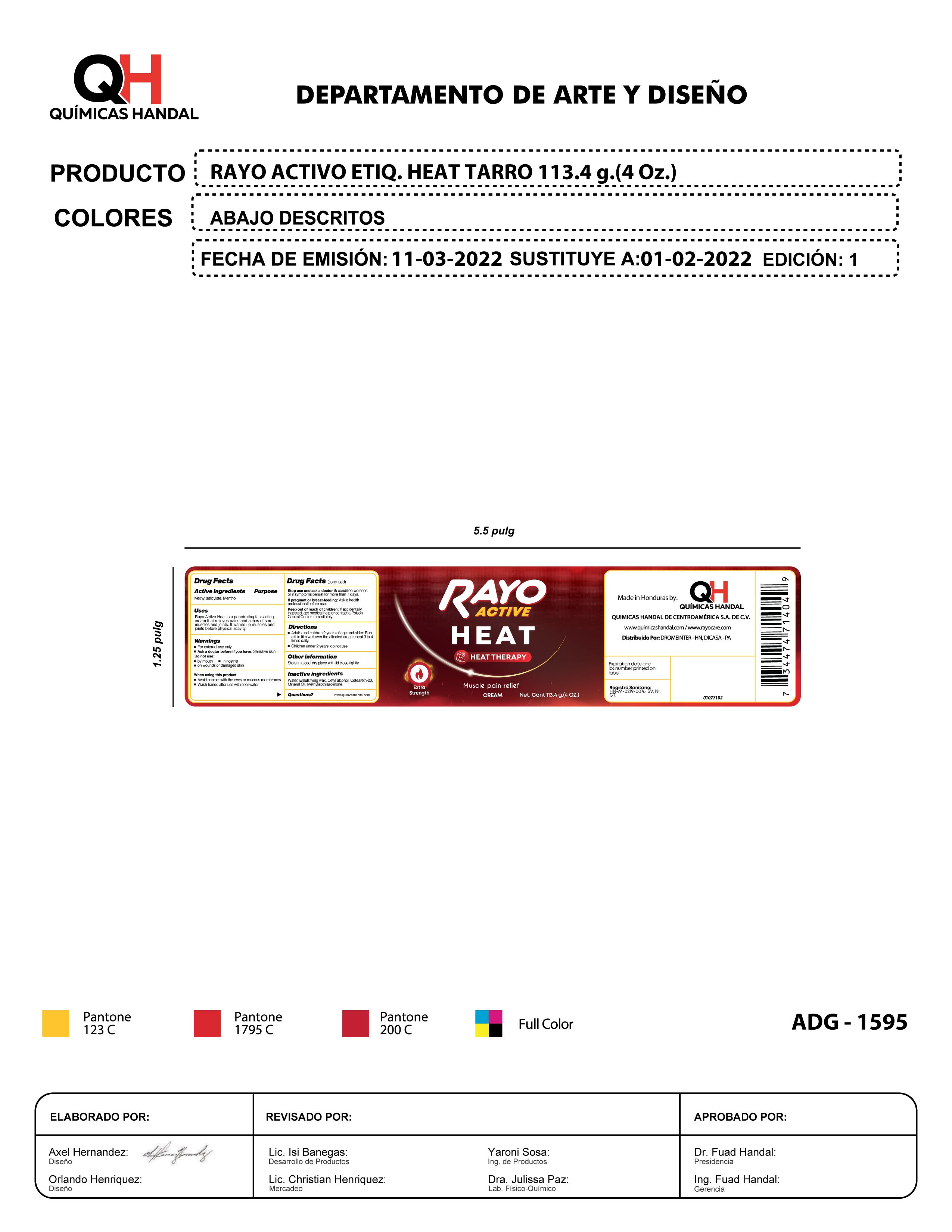
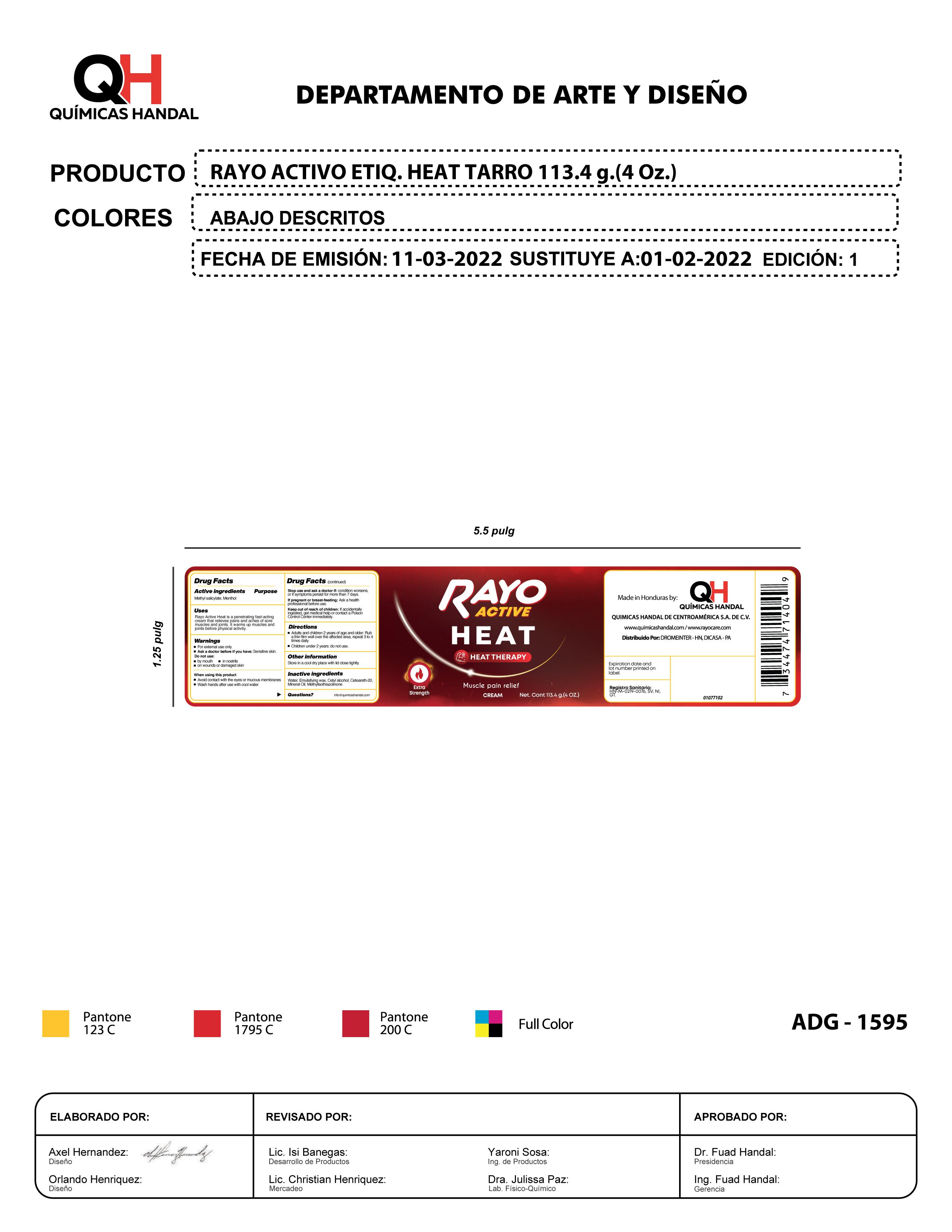
Label: RAYO ACTIVE HEAT- menthol, methyl salicylate cream
RAYO ACTIVE ICE- menthol, camphor cream
RAYO BREATH CHAMOMILE- menthol, camphor cream
RAYO BREATH MENTHOL- menthol, camphor cream
RAYO POWER ICE HEAT DUAL ACTION- menthol gel
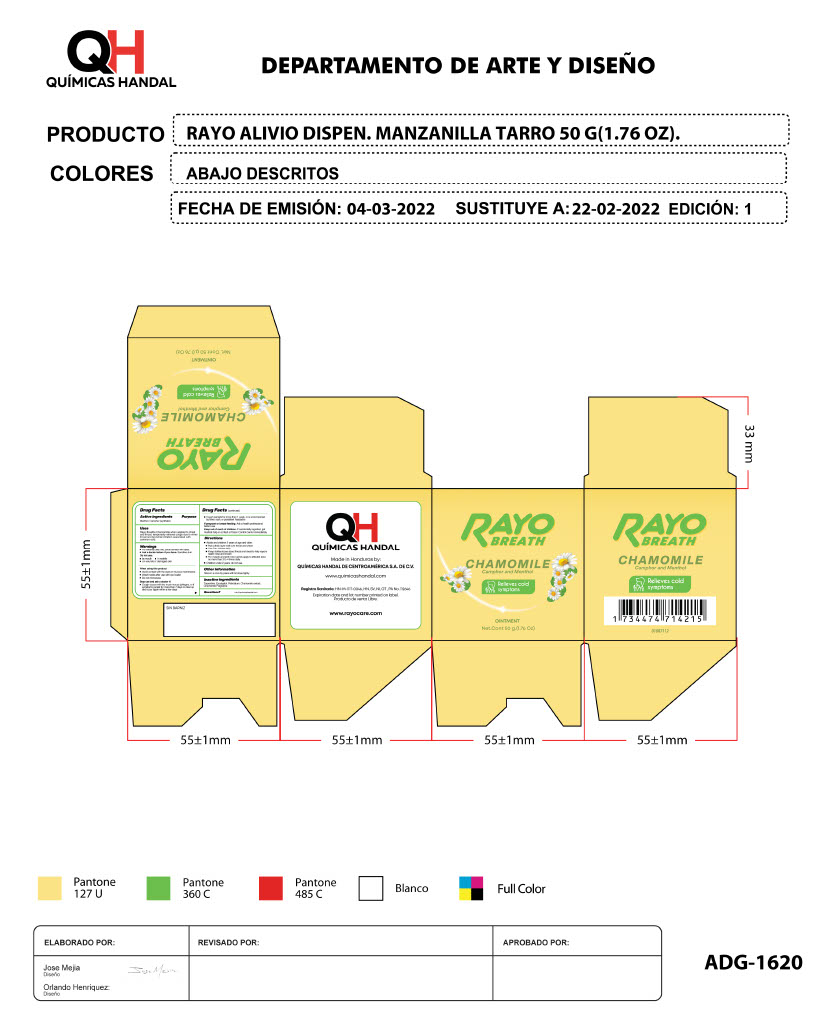
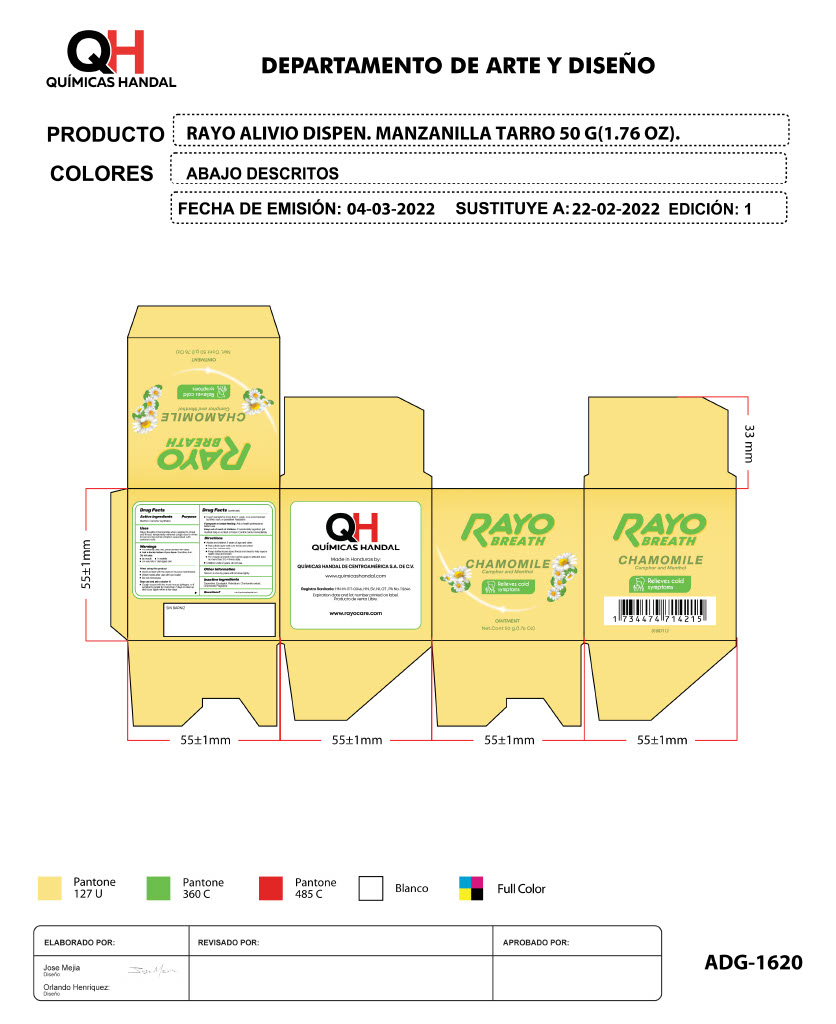
RAYO BREATH CHAMOMILE- menthol, camphor ointment
RAYO ACTIVE ICE SACHET- menthol, camphor cream
-
NDC Code(s):
83033-1594-1,
83033-1595-1,
83033-1598-1,
83033-1599-1, view more83033-1600-1, 83033-1601-1, 83033-1602-1, 83033-1605-1, 83033-1606-1, 83033-1609-1, 83033-1610-1, 83033-1611-1, 83033-1612-1, 83033-1620-1
- Packager: Quimicas Handal de Centroamerica SA de CV
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
Directions
- Adults and children 2 years of age and older:
- Rub a thick layer well over throat and chest
- Use 3 to 4 times daily
- Keep clothes loose about throat and chest to help vapors reach nose and mouth
- For muscle and joint minor aches apply to affected area no more than 3 to 4 times daily
- Children under 2 years: do not use.
-
WARNINGS
- For external use only; avoid contact with eyes
- Ask a doctor before if you have: sensitive skin
Do not use
- by mouth
- in nostrils
- on wounds or damaged skin
When using this product
- Avoid contact with the ayes or muccus membranes
- Wash hands after use with cool water
- Do not microwave
Stop use and ask a doctor if:
- Cough occurs with too much mucus (phlegm), or if symptoms persist for more than 7 days or clear up and occur again within a few days.
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
Directions
Adults and children 2 years of age and older:
Rub a thick layer well over throat and chest
Use 3 to 4 times daily
Keep clothes loose about throat and chest to help vapors reach nose and mouth
For muscle and joint minor aches apply to affected area no more than 3 to 4 times dailyChildren under 2 years: do not use.
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
- QUESTIONS
- 1620
- 1595
- 1606
- 1594
- 1602
- 1600
- 1598
- 1612
- 1609
- 1610
- 1605
- 1611
- 1599
- 1601
-
INGREDIENTS AND APPEARANCE
RAYO ACTIVE HEAT
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1610 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 1 g in 1 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g MINERAL OIL (UNII: T5L8T28FGP) 1 g in 1 g CETYL ALCOHOL (UNII: 936JST6JCN) 1 g in 1 g POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1610-1 1 in 1 BOX 11/01/2023 1 4 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 RAYO ACTIVE HEAT
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1595 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 1 g in 1 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g MINERAL OIL (UNII: T5L8T28FGP) 1 g in 1 g CETYL ALCOHOL (UNII: 936JST6JCN) 1 g in 1 g POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1595-1 1 in 1 BOX 11/01/2023 1 113.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 RAYO ACTIVE HEAT
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1599 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 1 g in 1 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g MINERAL OIL (UNII: T5L8T28FGP) 1 g in 1 g CETYL ALCOHOL (UNII: 936JST6JCN) 1 g in 1 g POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1599-1 1 in 1 BOX 11/01/2023 1 113.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 RAYO ACTIVE ICE
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g TROLAMINE (UNII: 9O3K93S3TK) 1 g in 1 g ISOPROPYL ALCOHOL (UNII: ND2M416302) 1 g in 1 g FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 1 g in 1 g CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1600-1 1 in 1 BOX 11/01/2023 1 226.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO BREATH CHAMOMILE
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1620 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength TURPENTINE (UNII: XJ6RUH0O4G) 1 g in 1 g EUCALYPTOL (UNII: RV6J6604TK) 1 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) 1 g in 1 g CHAMOMILE (UNII: FGL3685T2X) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1620-1 1 in 1 BOX 11/01/2023 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO ACTIVE ICE
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1594 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g TROLAMINE (UNII: 9O3K93S3TK) 1 g in 1 g ISOPROPYL ALCOHOL (UNII: ND2M416302) 1 g in 1 g FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 1 g in 1 g CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1594-1 1 in 1 BOX 11/01/2023 1 113.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO BREATH MENTHOL
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1605 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength TURPENTINE (UNII: XJ6RUH0O4G) 1 g in 1 g EUCALYPTOL (UNII: RV6J6604TK) 1 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1605-1 1 in 1 BOX 11/01/2023 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO BREATH MENTHOL
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1611 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength TURPENTINE (UNII: XJ6RUH0O4G) 1 g in 1 g EUCALYPTOL (UNII: RV6J6604TK) 1 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1611-1 1 in 1 BOX 11/01/2023 1 4 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO BREATH CHAMOMILE
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1612 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength TURPENTINE (UNII: XJ6RUH0O4G) 1 g in 1 g EUCALYPTOL (UNII: RV6J6604TK) 1 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) 1 g in 1 g CHAMOMILE (UNII: FGL3685T2X) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1612-1 1 in 1 BOX 11/01/2023 1 4 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO POWER ICE HEAT DUAL ACTION
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1602 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g TROLAMINE (UNII: 9O3K93S3TK) 1 g in 1 g ISOPROPYL ALCOHOL (UNII: ND2M416302) 1 g in 1 g CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1602-1 1 in 1 BOX 11/01/2023 1 80 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 RAYO BREATH CHAMOMILE
menthol, camphor ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1606 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength TURPENTINE (UNII: XJ6RUH0O4G) 1 g in 1 g EUCALYPTOL (UNII: RV6J6604TK) 1 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) 1 g in 1 g CHAMOMILE (UNII: FGL3685T2X) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1606-1 1 in 1 BOX 11/01/2023 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 RAYO ACTIVE ICE SACHET
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1609 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g TROLAMINE (UNII: 9O3K93S3TK) 1 g in 1 g ISOPROPYL ALCOHOL (UNII: ND2M416302) 1 g in 1 g FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 1 g in 1 g CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1609-1 1 in 1 BOX 11/01/2023 1 4 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 RAYO ACTIVE HEAT
menthol, methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 1 g in 1 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g MINERAL OIL (UNII: T5L8T28FGP) 1 g in 1 g CETYL ALCOHOL (UNII: 936JST6JCN) 1 g in 1 g POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1601-1 1 in 1 BOX 11/01/2023 1 226.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 RAYO ACTIVE ICE
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83033-1598 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1 g in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 g in 1 g WATER (UNII: 059QF0KO0R) 1 g in 1 g TROLAMINE (UNII: 9O3K93S3TK) 1 g in 1 g ISOPROPYL ALCOHOL (UNII: ND2M416302) 1 g in 1 g FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 1 g in 1 g CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) 1 g in 1 g METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83033-1598-1 1 in 1 BOX 11/01/2023 1 113.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2023 Labeler - Quimicas Handal de Centroamerica SA de CV (850638768) Registrant - Quimicas Handal de Centroamerica SA de CV (850638768) Establishment Name Address ID/FEI Business Operations Quimicas Handal de Centroamerica SA de CV 850638768 label(83033-1611, 83033-1620, 83033-1594, 83033-1595, 83033-1598, 83033-1599, 83033-1600, 83033-1601, 83033-1602, 83033-1605, 83033-1606, 83033-1609, 83033-1610, 83033-1612) , manufacture(83033-1620, 83033-1594, 83033-1595, 83033-1598, 83033-1599, 83033-1600, 83033-1601, 83033-1602, 83033-1605, 83033-1606, 83033-1609, 83033-1610, 83033-1611, 83033-1612)