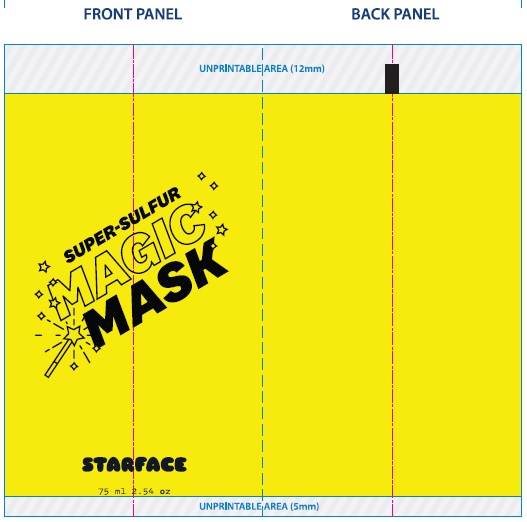
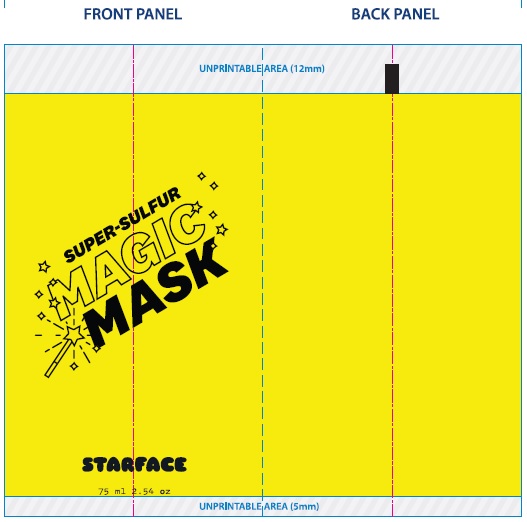
Label: SUPER-SULFUR MAGIC MASK- sulfur paste
- NDC Code(s): 83171-002-01
- Packager: Starface World, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
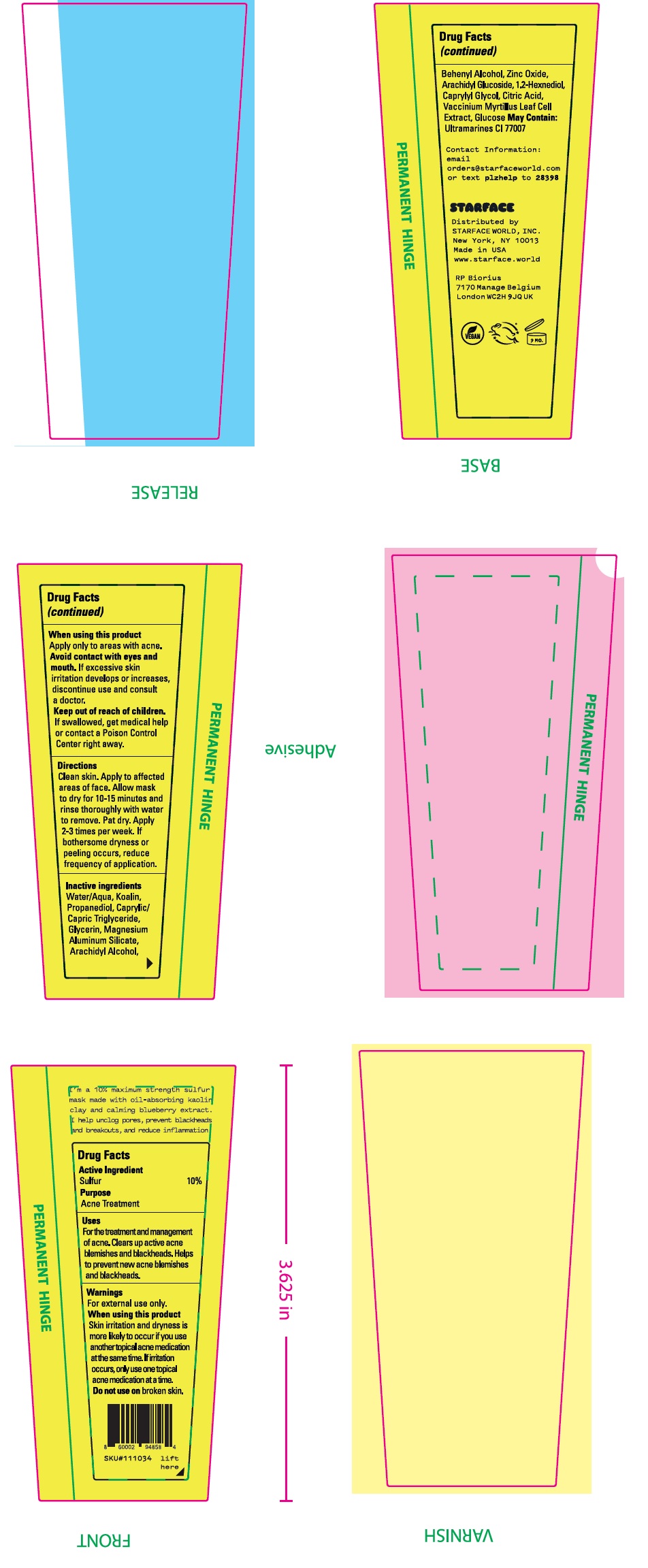
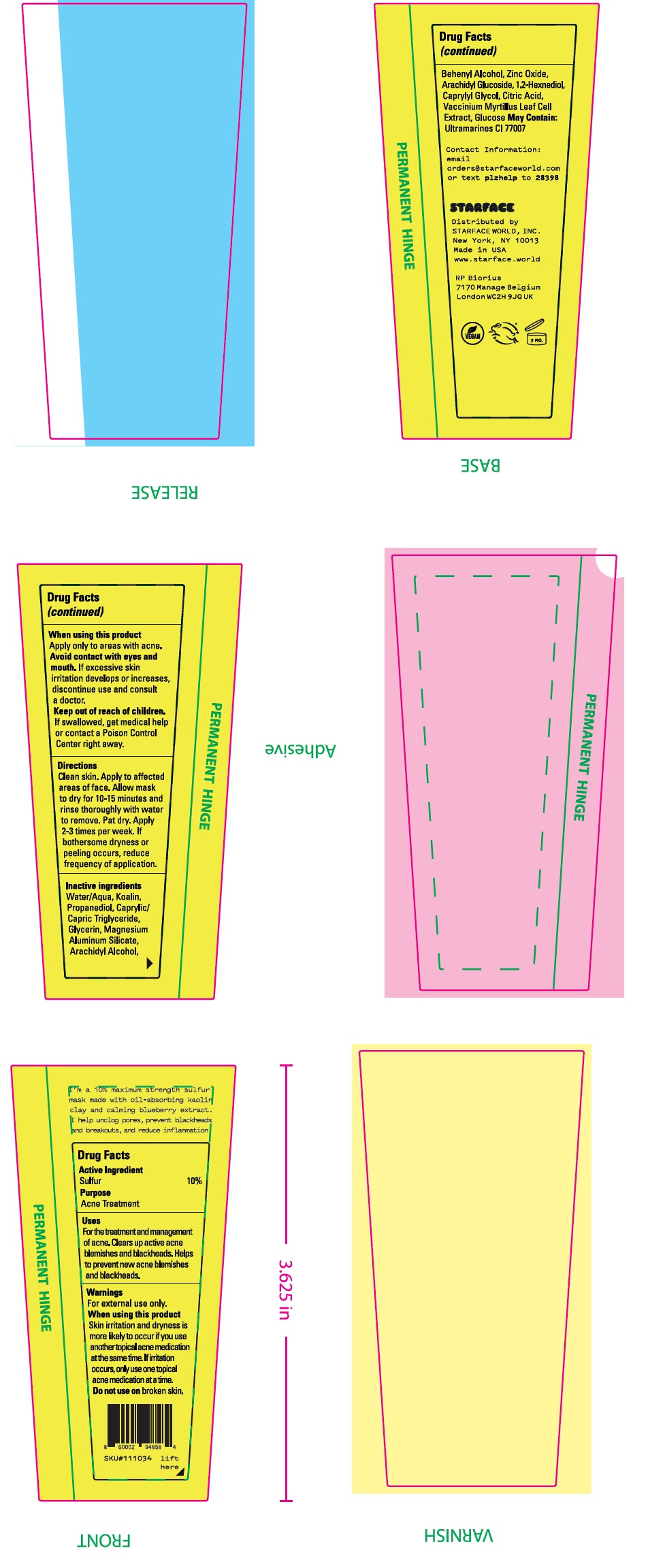
- Drug Facts
- Active Ingredients
- Uses:
-
Warnings
For external use only.
When using this product
Skin irritattion and dryness if more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Directions
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SUPER-SULFUR MAGIC MASK
sulfur pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83171-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) KAOLIN (UNII: 24H4NWX5CO) PROPANEDIOL (UNII: 5965N8W85T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) DOCOSANOL (UNII: 9G1OE216XY) ZINC OXIDE (UNII: SOI2LOH54Z) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) VACCINIUM MYRTILLUS LEAF (UNII: Y4U591OU70) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83171-002-01 75 mL in 1 TUBE; Type 0: Not a Combination Product 07/27/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 07/27/2022 Labeler - Starface World, Inc. (040399707)