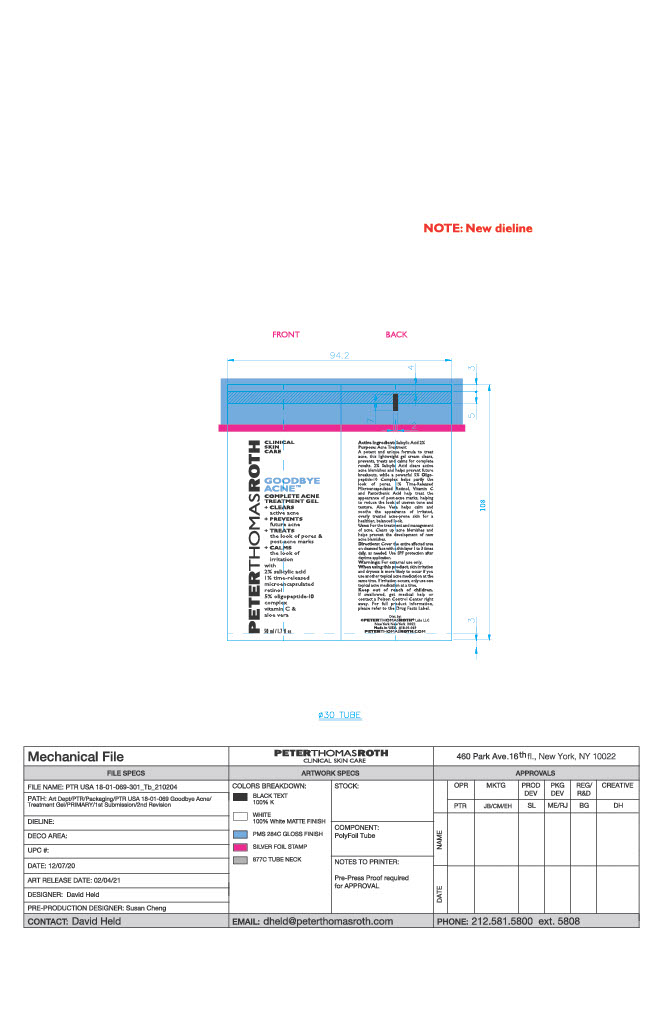
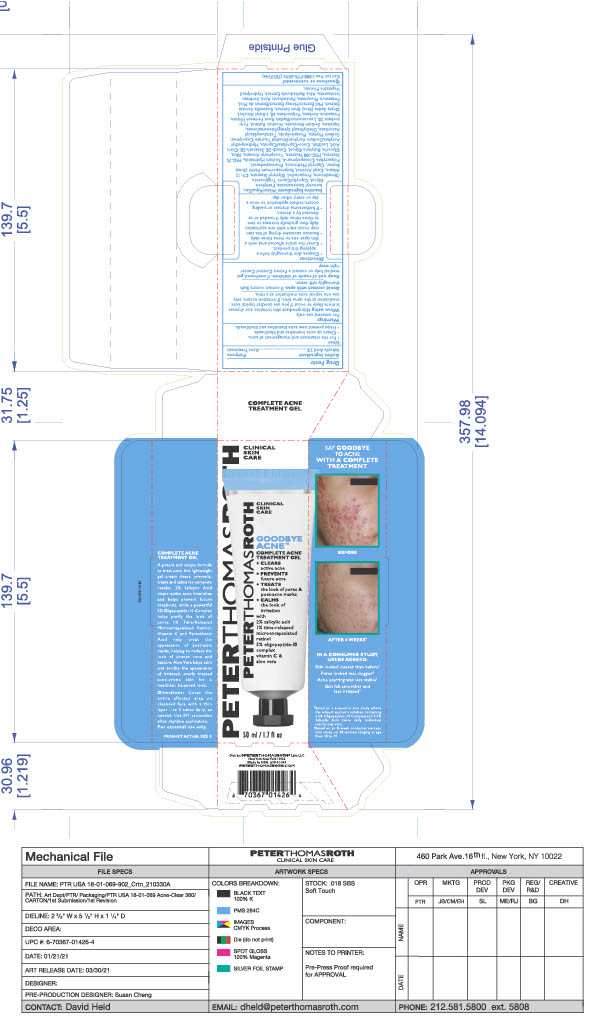
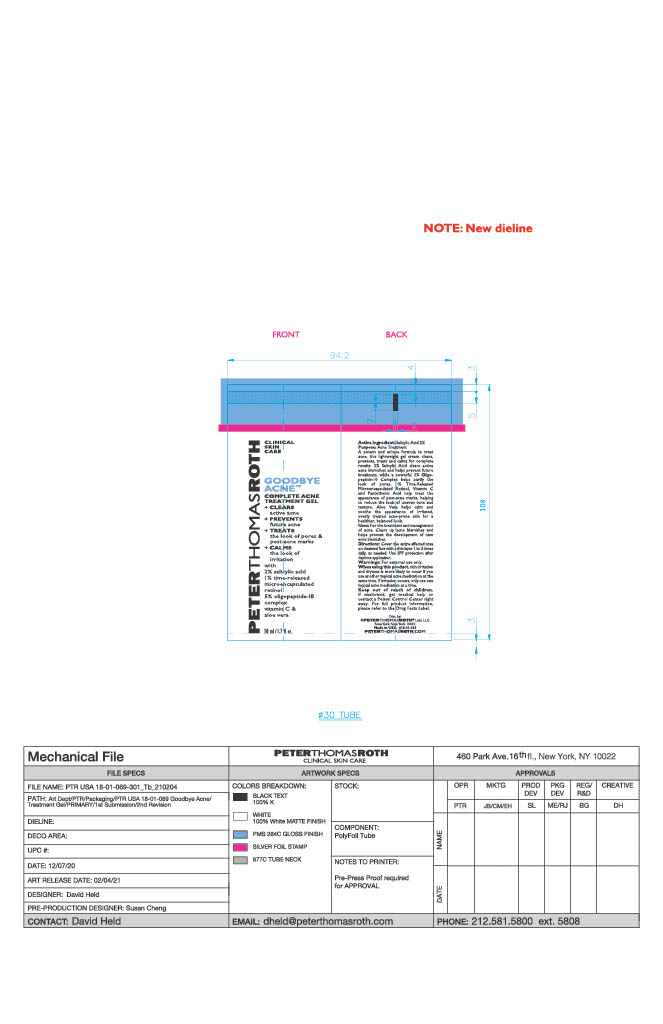
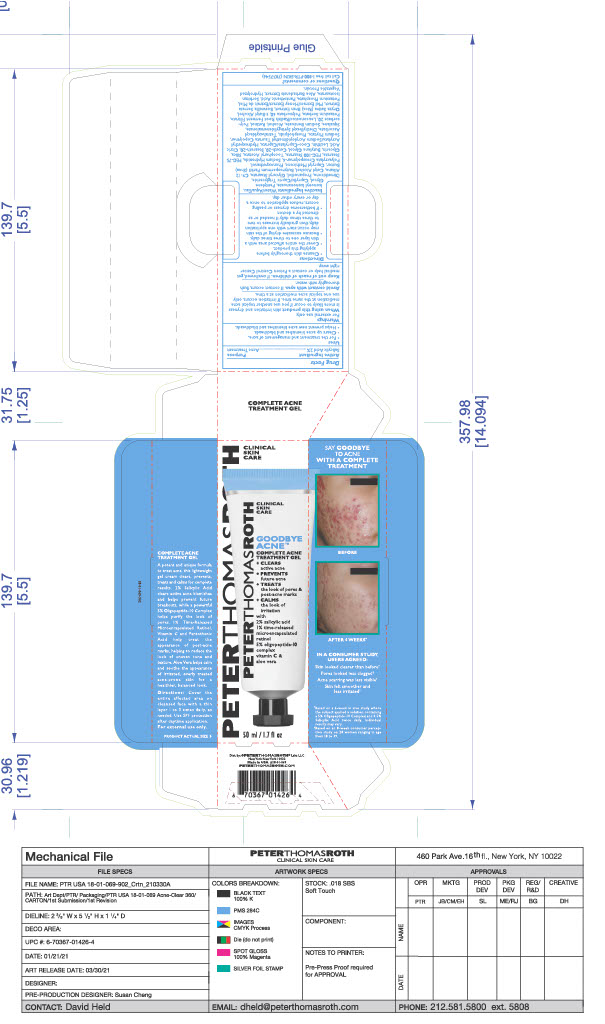
Label: GOODBYE ACNE COMPLETE TREATMENT GEL- salicylic acid gel
- NDC Code(s): 54031-069-01, 54031-069-02
- Packager: Peter Thomas Roth, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only.
When using this product skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Avoid contact with eyes. If contact occurs, flush thoroughly with water.
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- Cleanse skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, the gradually increase to two to three times daily if needed or as direrected by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INACTIVE INGREDIENT
Inactive Ingredients
Water/Aqua/Eau, Isononyl Isononanoate, Pentylene Glycol, Caprylic/Capric Triglyceride, Dimethicone, Propanediol, Glyceryl Stearate, C9-12 Alkane, Cetyl Alcohol, Butyrospermum Parkii (Shea) Butter, Caprylyl Methicone, Phenoxyethanol,Polyacrylate Crosspolymer-6, Sodium Hydroxide, PEG-75 Stearate, PEG-100 Stearate, Tocopheryl Acetate, Silica, Glycerin, Butylene Glycol, Ceteth-20, Steareth-20, Citric Acid, Lecithin, Coco-Caprylate/Caprate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Sodium Phytate, Phospholipids, Tetrahexyldecyl Ascorbate, Diethylhexyl Syringylidenemalonate, Squalane,Sodium Benzoate, Alcohol, Retinol, Polysorbate 20, Leuconostoc/Radish Root Ferment Filtrate, Potassium Sorbate, Polysorbate 60, t-Butyl Alcohol, Oryza Sativa (Rice) Bran Extract, Boswellia Serrata Extract, Honey Extract, Potassium Phosphate, Pantothenic Acid, Sorbitan Isostearate, Aloe Barbadensis Extract, Hydrolyzed Vegetable Protein
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOODBYE ACNE COMPLETE TREATMENT GEL
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54031-069 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.9638 g in 48.19 g Inactive Ingredients Ingredient Name Strength PEG-75 STEARATE (UNII: OT38R0N74H) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) ALCOHOL (UNII: 3K9958V90M) HONEY (UNII: Y9H1V576FH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIMETHICONE (UNII: 92RU3N3Y1O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) WATER (UNII: 059QF0KO0R) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) C9-12 ALKANE (UNII: 7J5R5W72QM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYSORBATE 20 (UNII: 7T1F30V5YH) SHEA BUTTER (UNII: K49155WL9Y) PENTYLENE GLYCOL (UNII: 50C1307PZG) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHYTATE SODIUM (UNII: 88496G1ERL) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) RICE BRAN (UNII: R60QEP13IC) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) RETINOL (UNII: G2SH0XKK91) POLYSORBATE 60 (UNII: CAL22UVI4M) CETETH-20 (UNII: I835H2IHHX) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) STEARETH-20 (UNII: L0Q8IK9E08) SQUALANE (UNII: GW89575KF9) SODIUM BENZOATE (UNII: OJ245FE5EU) ALOE (UNII: V5VD430YW9) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PANTOTHENIC ACID (UNII: 19F5HK2737) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54031-069-01 48.19 g in 1 TUBE; Type 0: Not a Combination Product 06/15/2021 2 NDC:54031-069-02 48.19 g in 1 CARTON; Type 0: Not a Combination Product 06/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/15/2021 Labeler - Peter Thomas Roth, LLC (780458944) Registrant - June Jacobs Labs, LLC (122610681) Establishment Name Address ID/FEI Business Operations June Jacobs Labs, LLC 122610681 manufacture(54031-069)