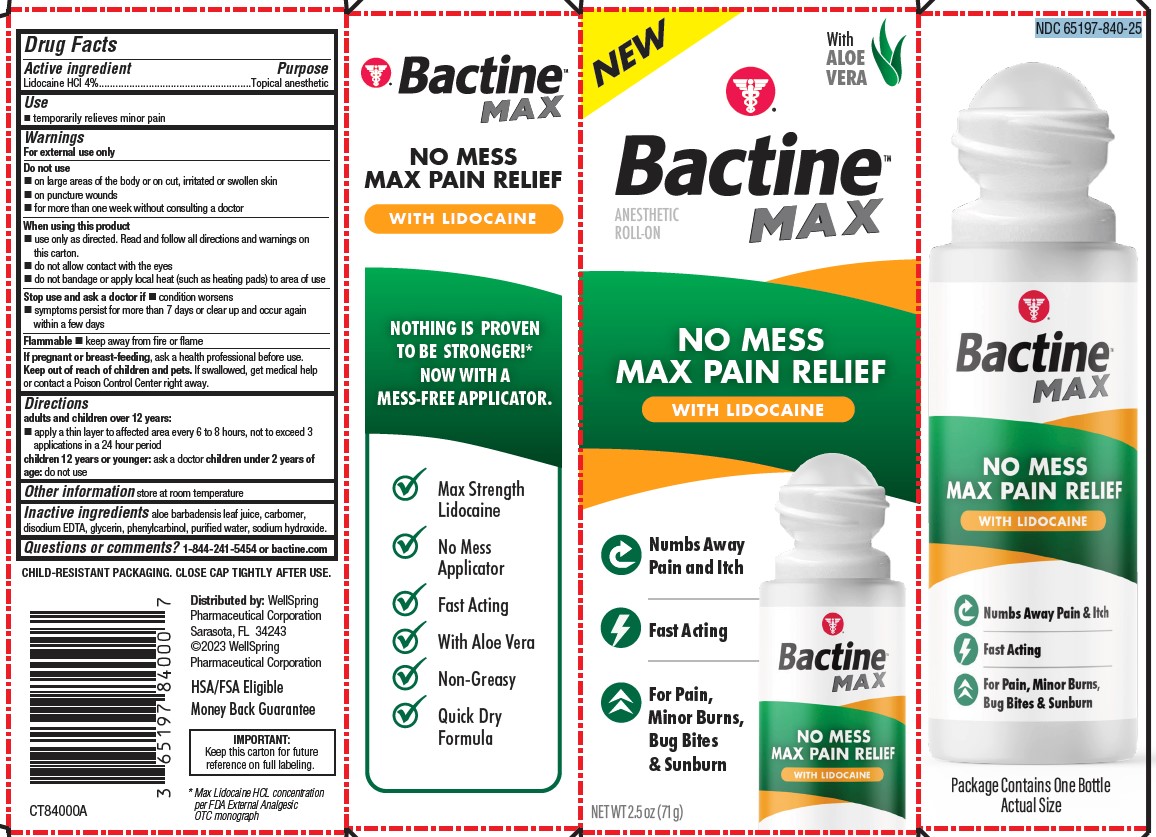
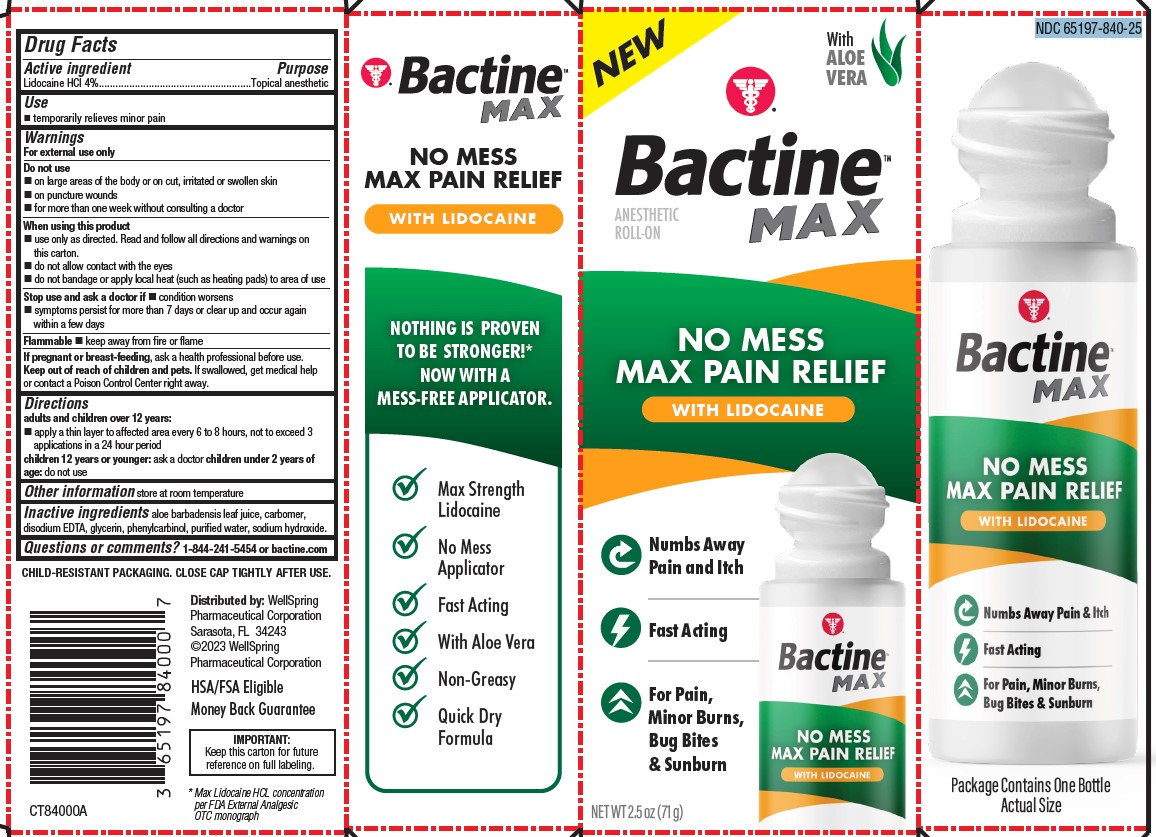
Label: BACTINE MAX LIDOCAINE ROLL-ON 2.5OZ- lidocaine liquid
- NDC Code(s): 65197-840-25
- Packager: WellSpring Pharmaceutical Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (w/w)
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- do not allow contact with the eyes
- do not bandage or apply local heat (such as heating pads) to area of use
- Directions
- Other information
- Inactive ingredients
- Questions?
- Distributed by:
-
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
NO MESS MAX PAIN RELIEF WITH LIDOCAINE
Numbs Away Pain and Itch
Fast Acting
For Pain, Minor Burns, Bug Bites & Sunburn
Max Strength Lidocaine
No Mess Applicator
Fast Acting
With Aloe Vera
Non-Greasy
Quick Dry Formula
HSA/FSA Eligible
MONEY BACK GUARANTEE
IMPORTANT:
Keep this carton for future reference on full labeling.* Max Lidocaine HCL concentration per FDA External Analgesic OTC monograph
NDC 65197-840-25
Bactine Max Roll-on Artwork
Back Panel
New
Bactine Max
Liquid Bandage with Lidocaine
SHAKE WELL
Bactine Max Liquid Bandageʼs unique formula provides maximum strength pain relief with LIDOCAINE, kills 99.9% of germs*, and protects hard-to-cover minor cuts and scrapes. This flexible bandage dries clear quickly and is breathable to maximize healing.
-
INGREDIENTS AND APPEARANCE
BACTINE MAX LIDOCAINE ROLL-ON 2.5OZ
lidocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65197-840 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) Product Characteristics Color white (translucent) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65197-840-25 1 in 1 BOX 01/15/2024 1 71 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/15/2024 Labeler - WellSpring Pharmaceutical Corporation (110999054)