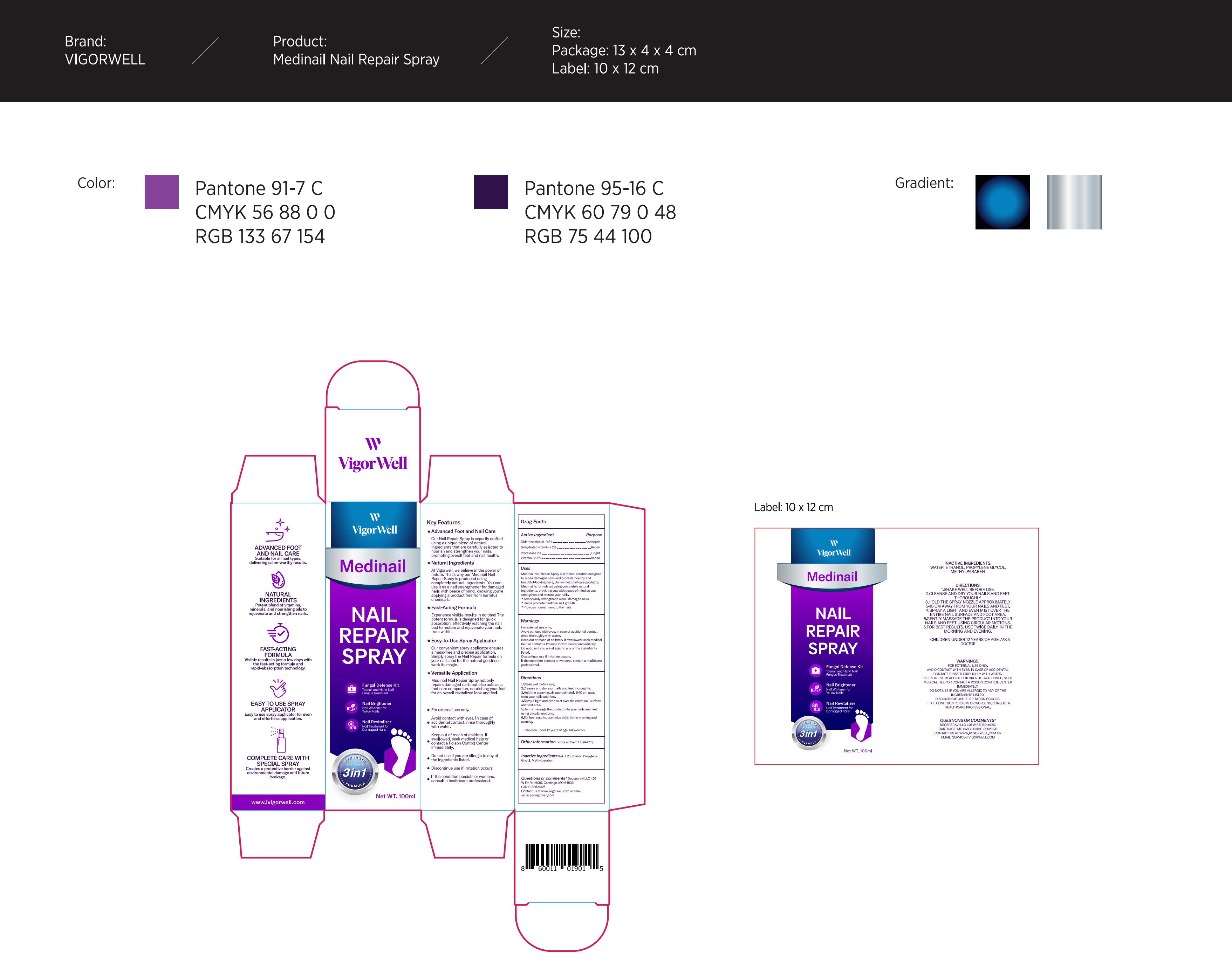
Label: VIGORWELL MEDINAIL TOENAIL FUNGUS TREATMENT SPRAY.- toenail spray liquid
- NDC Code(s): 82739-017-01, 82739-017-02, 82739-017-03, 82739-017-04
- Packager: Shenzhen Situya Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Use
Shake well before use.
Cleanse and dry your nails and feet thoroughly.
Hold the spray nozzle approximately 5-10 cm away from your nails and feet.
Spray a light and even mist over the entire nail surface and foot area.
Gently massage the product into your nails and feet using circular motions.
For best results, use twice daily, in the morning and evening. - Warnings
- Do not use
- When Using
- Stop Use
- Keep Out Of Reach Of Children
-
Directions
1.Shake well before use.
2.Cleanse and dry your nails and feet thoroughly.
3.Hold the spray nozzle approximately 5-10 cm awayfrom your nails and feet.
4.Spray a light and even mist over the entire nail surfaceand foot area.
5.Gently massage the product into your nails and feetusing circular motions.
6.For best results, use twice daily, in the morning andevening.
- Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIGORWELL MEDINAIL TOENAIL FUNGUS TREATMENT SPRAY.
toenail spray liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82739-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 2 g in 100 mL CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE ACETATE 0.2 g in 100 mL PROQUAZONE (UNII: 42VPJ2980S) (PROQUAZONE - UNII:42VPJ2980S) PROQUAZONE 2 g in 100 mL ANHYDROVITAMIN A (UNII: 235BBF3K97) (ANHYDROVITAMIN A - UNII:235BBF3K97) ANHYDROVITAMIN A 2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82739-017-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/07/2023 2 NDC:82739-017-02 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/07/2023 3 NDC:82739-017-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/07/2023 4 NDC:82739-017-04 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 11/07/2023 Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) Establishment Name Address ID/FEI Business Operations Shenzhen Situya Trading Co., Ltd. 706154255 label(82739-017) , manufacture(82739-017)