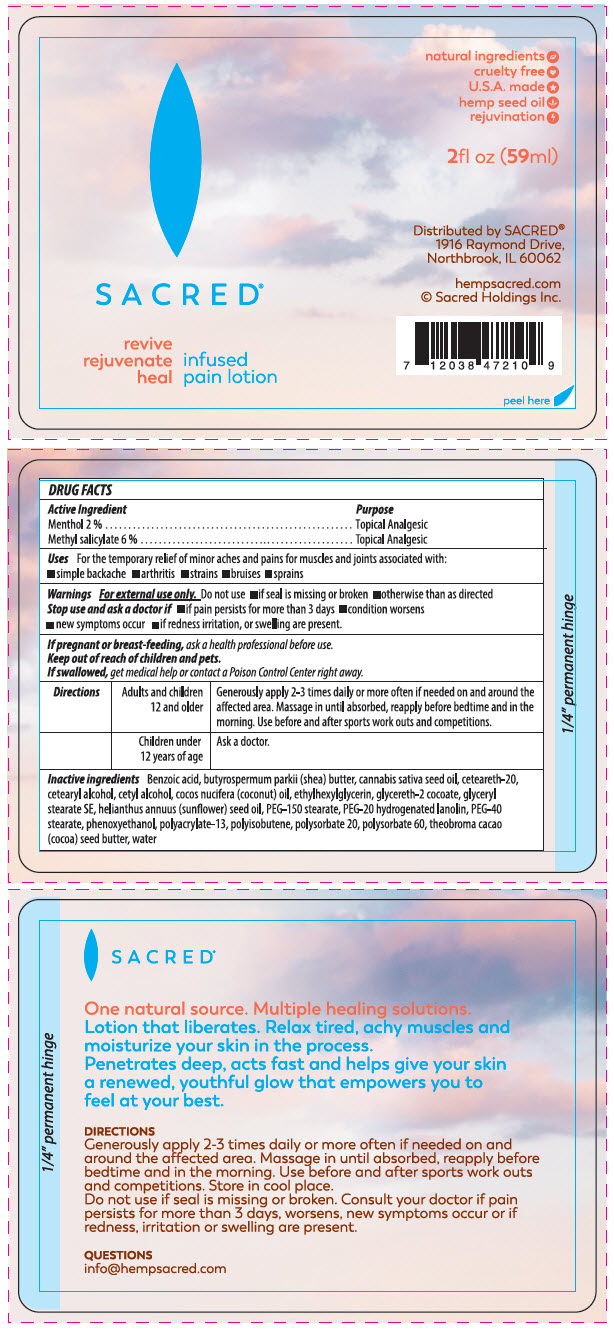
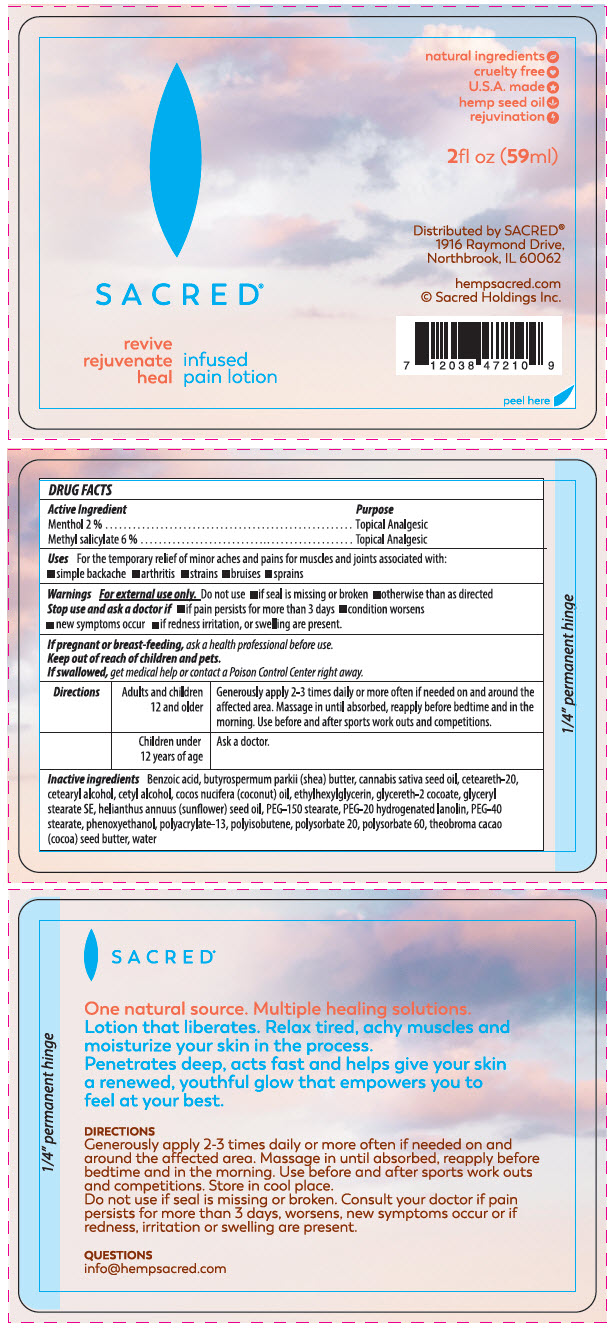
Label: HEMP PAIN- menthol, unspecified form and methyl salicylate lotion
- NDC Code(s): 73616-210-02
- Packager: Sacred Enterprises, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 24, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
DOSAGE & ADMINISTRATION
Directions Adults and children 12 and older Generously apply 2-3 times daily or more often if needed on and around the affected area. Massage in until absorbed, reapply before bedtime and in the morning. Use before and after sports work outs and competitions. Children under 12 years of age Ask a doctor. -
Inactive ingredients
Benzoic acid, Butyrospermum parkii ( shea) butter, Cannabis sativa seed oil, Ceteareth-20,cetearyl alcohol, Cetyl alcohol, Cocos Nucifera ( coconut) oil, Ethylhexylglycerin, Glycereth 2 cocoate, Glyceryl stearate SE, Helianthus annuus ( sunflower) seed oil , PEG -150 stearate, PEG-20 hydrogenated lanolin, PEG-40 stearate, Phenoxyethanol polyacrulate-13, Polyisobutene, Polysorbate 20, Polysorbate 60, Theobroma cacao ( cocoa) seed butter, Water.
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 59 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
HEMP PAIN
menthol, unspecified form and methyl salicylate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73616-210 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 2 mg in 100 mL Methyl Salicylate (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) Methyl Salicylate 6 mg in 100 mL Inactive Ingredients Ingredient Name Strength SHEA BUTTER (UNII: K49155WL9Y) Benzoic Acid (UNII: 8SKN0B0MIM) Cannabis Sativa Seed Oil (UNII: 69VJ1LPN1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) Cetyl Alcohol (UNII: 936JST6JCN) COCONUT OIL (UNII: Q9L0O73W7L) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERETH-20 COCOATE (UNII: 38RO881Z06) Glyceryl Stearate SE (UNII: FCZ5MH785I) SUNFLOWER OIL (UNII: 3W1JG795YI) Peg-20 Hydrogenated Lanolin (UNII: 5PP3KJ4T6S) Peg-40 Stearate (UNII: ECU18C66Q7) PEG-150 STEARATE (UNII: 7BSG7DF10Q) Phenoxyethanol (UNII: HIE492ZZ3T) POLYACRYLAMIDE (1300000 MW) (UNII: SC5Y4X78TG) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) Polysorbate 20 (UNII: 7T1F30V5YH) Polysorbate 60 (UNII: CAL22UVI4M) COCOA BUTTER (UNII: 512OYT1CRR) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73616-210-02 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 09/01/2019 Labeler - Sacred Enterprises, LLC (094283990) Registrant - BMC 1092, Inc dba Solo Labs Inc. (078831987) Establishment Name Address ID/FEI Business Operations BMC 1092, Inc dba Solo Labs Inc. 078831987 MANUFACTURE(73616-210) , PACK(73616-210)