Label: MQ PAIN RELIEVING PATCH- pain relieving patch patch
- NDC Code(s): 83781-006-01, 83781-006-02, 83781-006-03
- Packager: Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
Warnings For external use only
Do not use:★on wounds or damaged skin
★if you are allergic to aspirin or salicylates.
Stop use and ask a doctor if:★ Conditions worsen.
★ Symptoms persist for more than 7 days.
★Symptoms clear up and occur again within a few days.★Rash, itching or excessive skin irritation occurs.
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Out Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- Questions
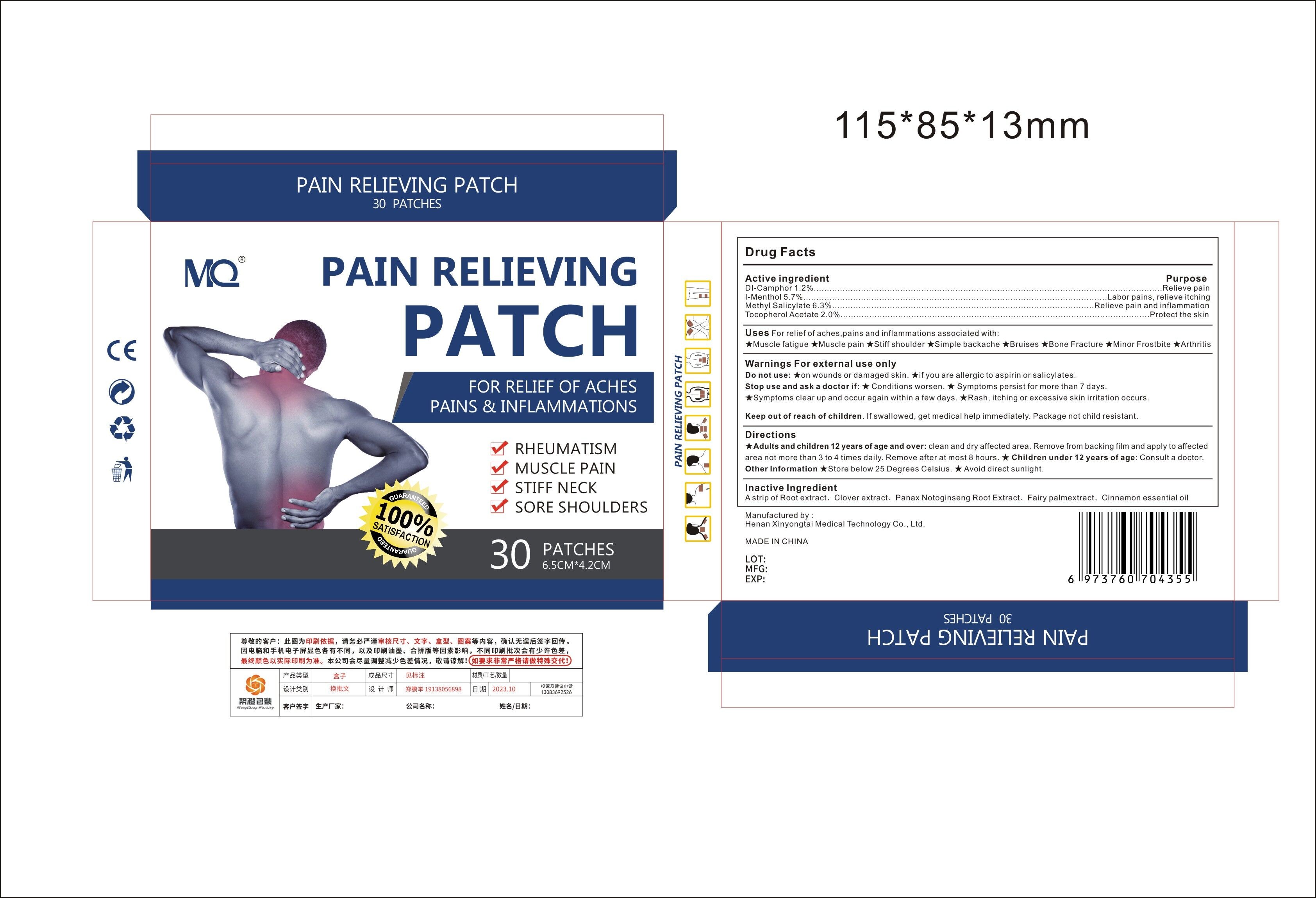
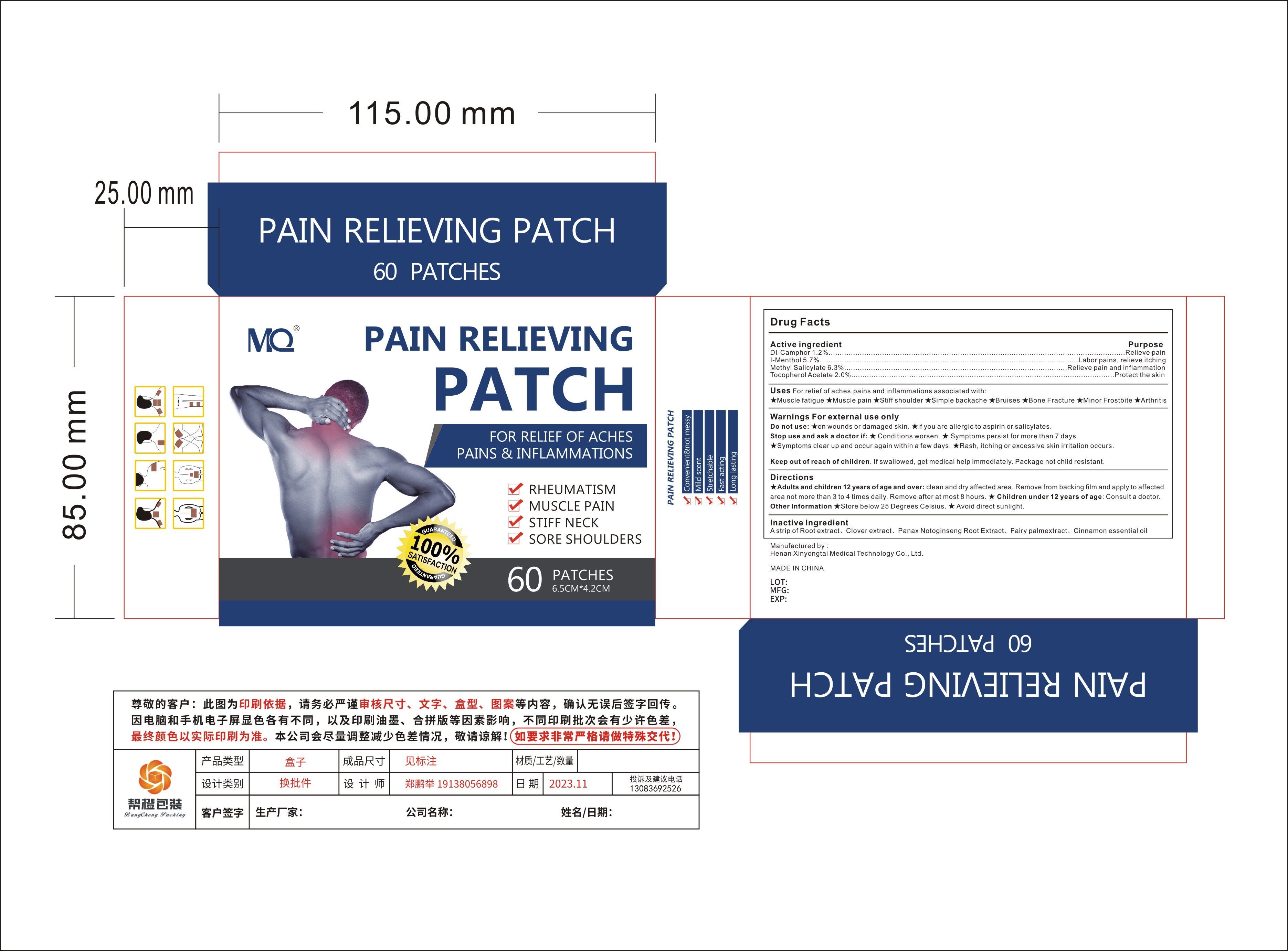
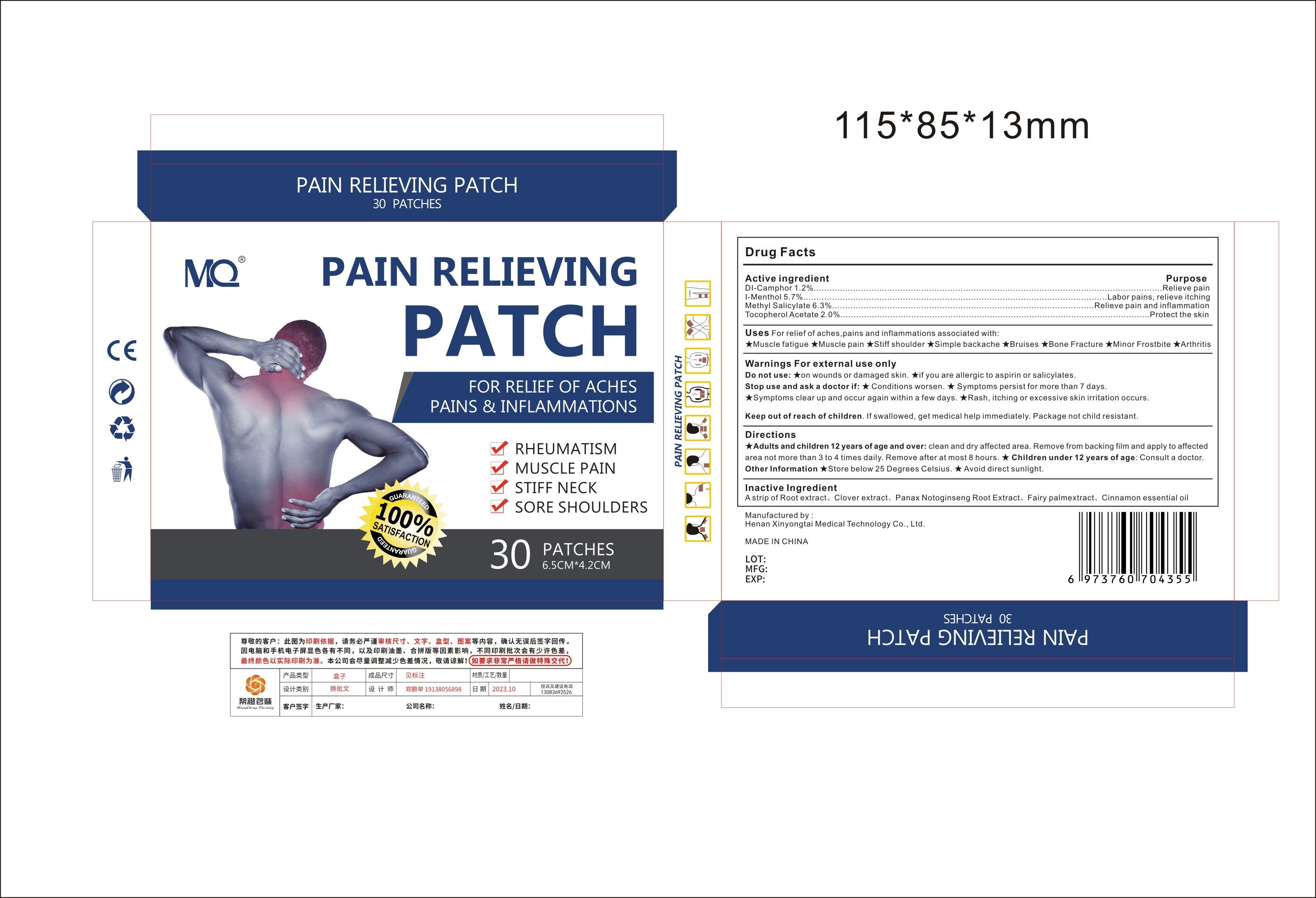
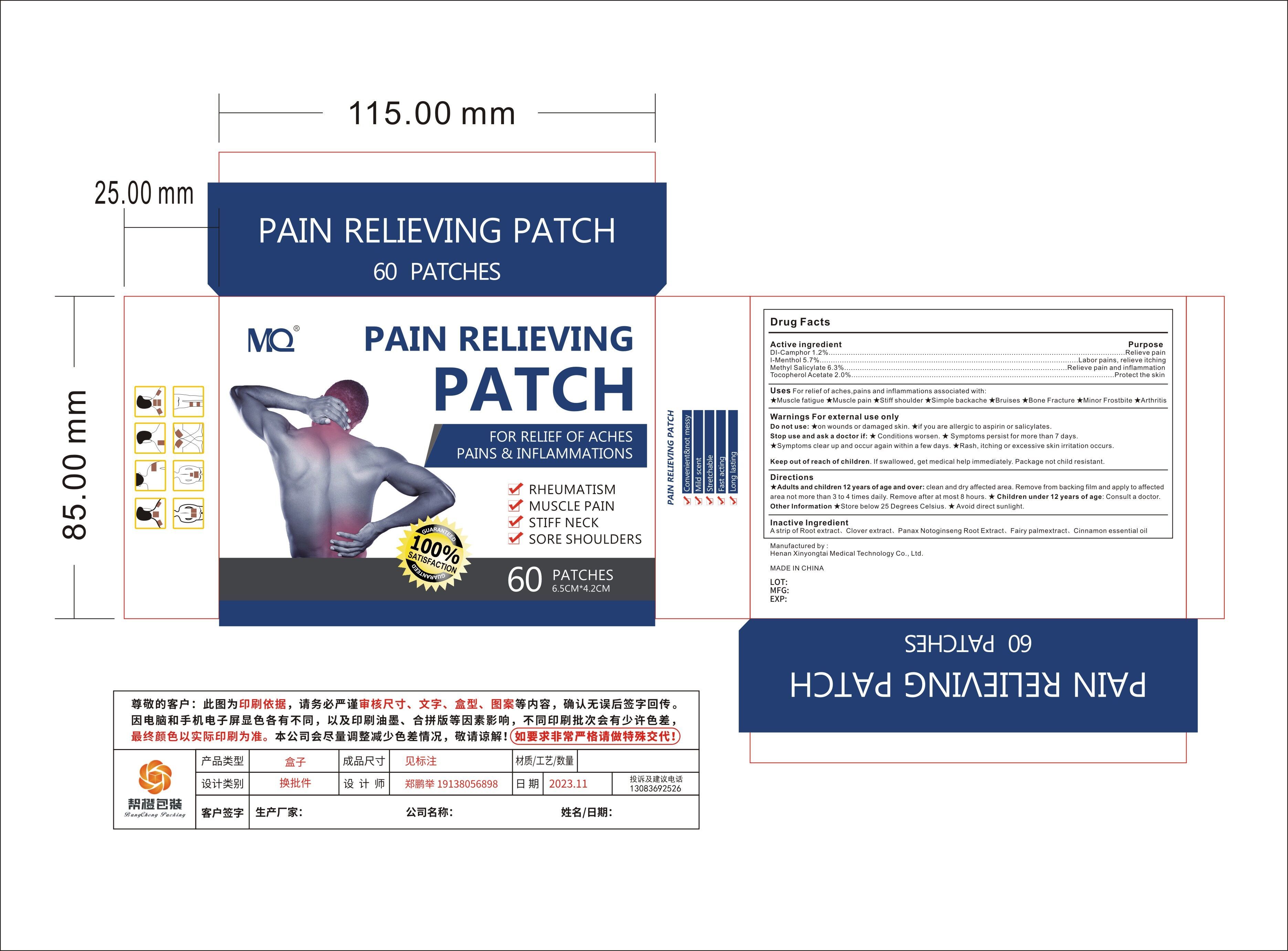
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MQ PAIN RELIEVING PATCH
pain relieving patch patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83781-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 5.7 g in 100 METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 6.3 g in 100 CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1.2 g in 100 .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 2 g in 100 Inactive Ingredients Ingredient Name Strength GYNOSTEMMA PENTAPHYLLUM LEAF (UNII: 5DWS84R85M) FLEMINGIA PROSTRATA WHOLE (UNII: R0GG9QUA0Q) CLOVE (UNII: K48IKT5321) PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) CINNAMON OIL (UNII: E5GY4I6YCZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83781-006-01 30 in 1 BOX; Type 0: Not a Combination Product 11/05/2023 2 NDC:83781-006-02 60 in 1 BOX; Type 0: Not a Combination Product 11/05/2023 3 NDC:83781-006-03 240 in 1 BOX; Type 0: Not a Combination Product 11/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/05/2023 Labeler - Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd (701762807) Establishment Name Address ID/FEI Business Operations Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd 701762807 label(83781-006) , manufacture(83781-006)