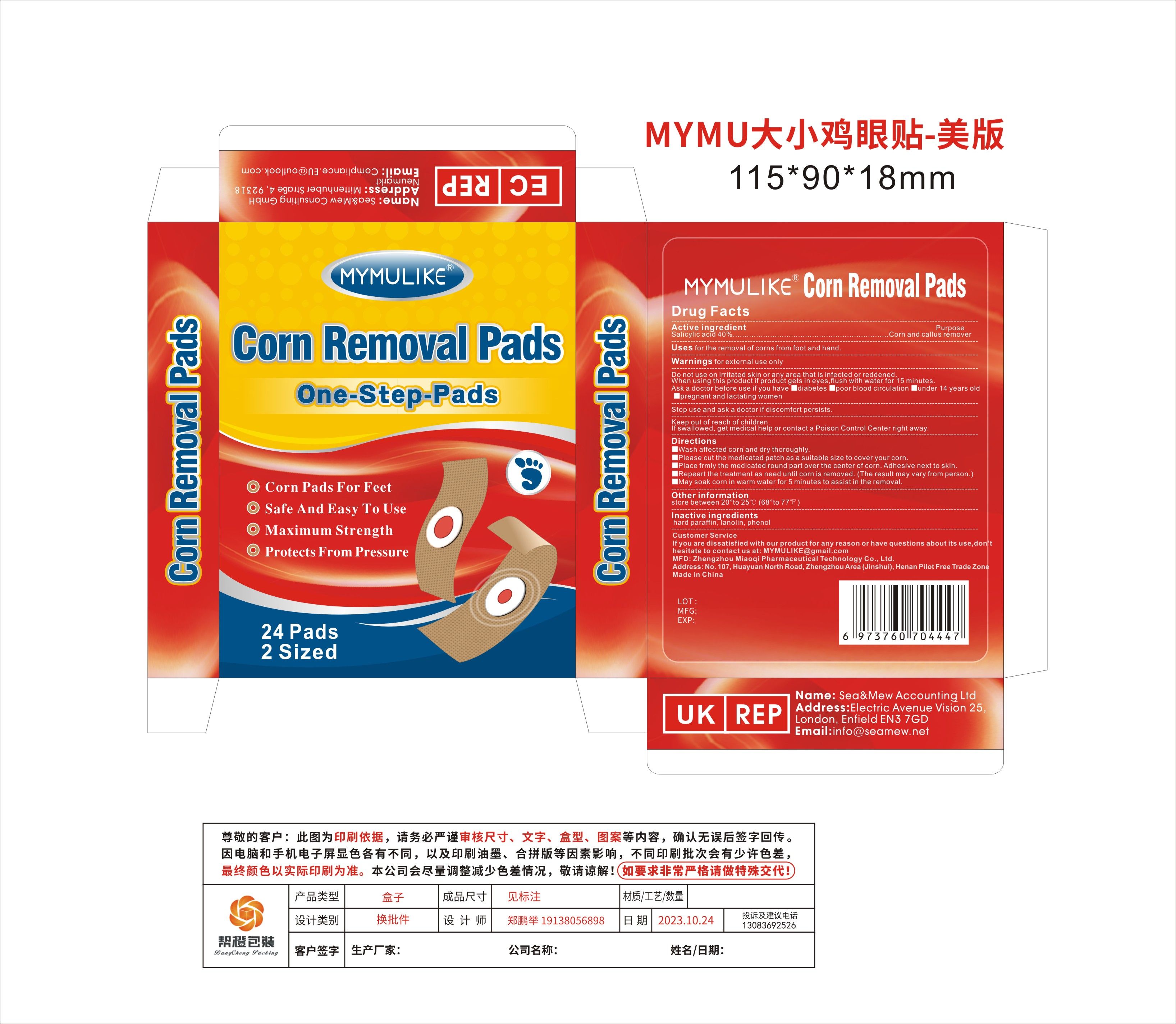
Label: MYMULIKE CORN REMOVAL PADS- corn removal pads patch
- NDC Code(s): 83781-003-01
- Packager: Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Out Of Reach Of Children
-
Directions
■Wash affected corn and dry thoroughly.
■Please cut the medicated patch as a suitable size to cover your corn.
■Place frmly the medicated round part over the center of corn. Adhesive next to skin.
■Repeart the treatment as need until corn is removed. (The result may vary from person.)
■May soak corn in warm water for 5 minutes to assist in the removal.
- Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MYMULIKE CORN REMOVAL PADS
corn removal pads patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83781-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 g in 100 Inactive Ingredients Ingredient Name Strength PARAFFIN (UNII: I9O0E3H2ZE) PHENOL (UNII: 339NCG44TV) LANOLIN (UNII: 7EV65EAW6H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83781-003-01 24 in 1 BOX; Type 0: Not a Combination Product 11/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M030 11/05/2023 Labeler - Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd (701762807) Establishment Name Address ID/FEI Business Operations Zhengzhou Miaoqi Pharmaceutical Technology Co., Ltd 701762807 label(83781-003) , manufacture(83781-003)