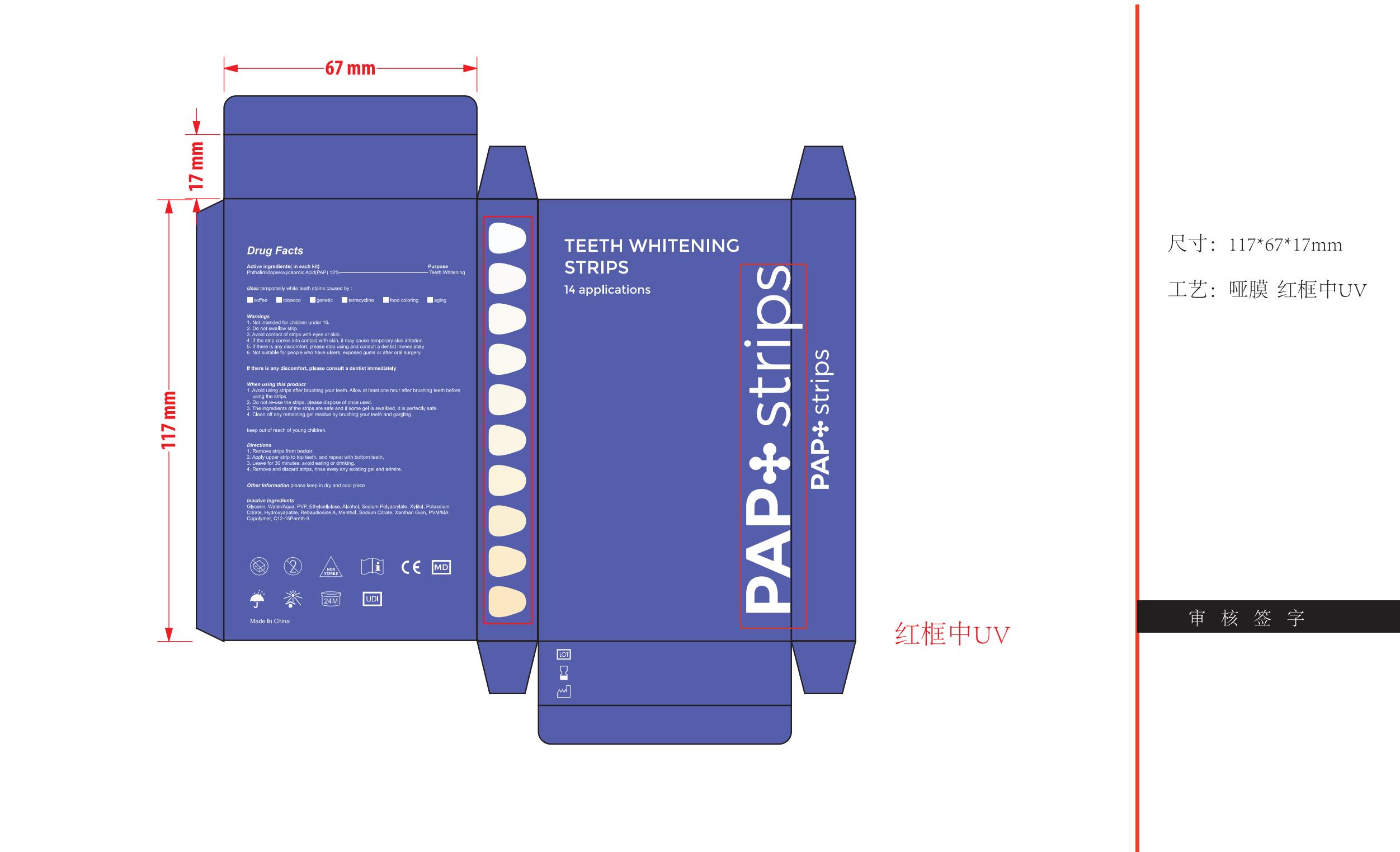
Label: TEETH WHITENING STRIPS patch
- NDC Code(s): 83778-003-01
- Packager: Nanchang Dental Bright Technology Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
1. Not intended for children under 16.
2. Do not swallow strip.
3. Avoid contact of strips with eyes or skin.
4. If the strip comes into contact with skin, it may cause temporary skin irritation.
5. If there is any discomfort, please stop using and consult a dentist immediately
6. Not suitable for people who have ulcers, exposed gums or after oral surgery. - Do not use
-
When Using
1. Avoid using strips after brushing your teeth. Allow at least one hour after brushing teeth before using the strips.
2. Do not re-use the strips, please dispose of once used.
3. The ingredients of the strips are safe and if some gel is swalloed, it is perfectly safe.
4. Clean off any remaining gel residue by brushing your teeth and gargling. - Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEETH WHITENING STRIPS
teeth whitening strips patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83778-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHTHALIMIDOPEROXYCAPROIC ACID (UNII: 5OEJ6FAL6C) (PHTHALIMIDOPEROXYCAPROIC ACID - UNII:5OEJ6FAL6C) PHTHALIMIDOPEROXYCAPROIC ACID 12 g in 100 Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) POTASSIUM CITRATE (UNII: EE90ONI6FF) MENTHOL (UNII: L7T10EIP3A) SODIUM CITRATE (UNII: 1Q73Q2JULR) GLYCERIN (UNII: PDC6A3C0OX) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) METHYL VINYL ETHER AND MALEIC ANHYDRIDE COPOLYMER (1100000 WAMW) (UNII: T0VRI38HB0) C12-15 PARETH-3 (UNII: 459EF9MP3Y) POVIDONE (UNII: FZ989GH94E) ALCOHOL (UNII: 3K9958V90M) REBAUDIOSIDE A (UNII: B3FUD0528F) WATER (UNII: 059QF0KO0R) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83778-003-01 14 in 1 BOX; Type 0: Not a Combination Product 11/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/05/2023 Labeler - Nanchang Dental Bright Technology Co.,Ltd. (544503502) Establishment Name Address ID/FEI Business Operations Nanchang Dental Bright Technology Co.,Ltd. 544503502 label(83778-003) , manufacture(83778-003)