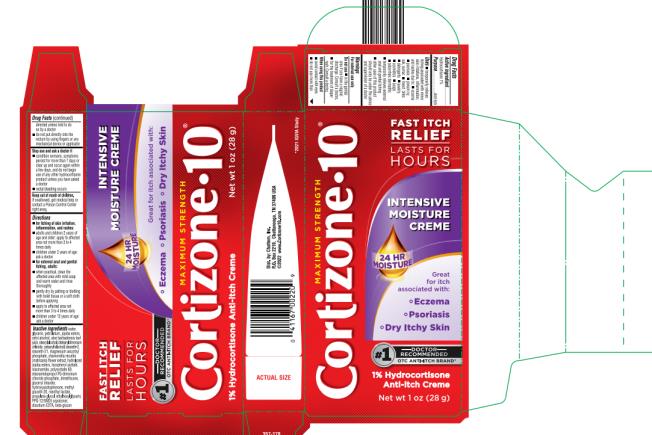
Label: CORTISONE 10 INTENSIVE MOISTURE CREME- hydrocortisone cream
- NDC Code(s): 41167-0022-0, 41167-0022-5, 41167-0022-6
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
-
Uses
■ temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
■ eczema ■ psoriasis ■ poison ivy, oak, sumac ■ insect bites ■ detergents ■ jewelry
■ cosmetics ■ soaps ■ seborrheic dermatitis
■ temporarily relieves external anal and genital itching
■ other uses of this product should only be under the advice and supervision of a doctor
-
Warnings
For external use only
Do not use
■ in the genital area if you have a vaginal discharge. Consult a doctor. ■ for the treatment of diaper rash. Consult a doctor.
When using this product
■ avoid contact with eyes ■ do not use more than directed unless told to do so by a doctor ■ do not put directly into the rectum by using fingers or any mechanical device or applicator
-
Directions
■ for itching of skin irritation, inflammation, and rashes:
■ adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
■ children under 2 years of age: ask a doctor
■ for external anal and genital itching, adults:
■ when practical, clean the affected area with mild soap and warm water and rinse thoroughly
■ gently dry by patting or blotting with toilet tissue or a soft cloth before applying
■ apply to affected area not more than 3 to 4 times daily
■ children under 12 years of age: ask a doctor
-
Inactive ingredients
water, glycerin, petrolatum, jojoba esters, cetyl alcohol, aloe barbadensis leaf juice, stearyl alcohol, distearyldimonium chloride, cetearyl alcohol, steareth-2, steareth-21, magnesium ascorbyl phosphate, chamomilla recutita (matricaria) flower extract, hydrolyzed jojoba esters, tocopheryl acetate, niacinamide, polysorbate 60, stearamidopropyl PG-dimonium chloride phosphate, dimethicone, glyceryl stearate, hydroxyacetophenone, methyl gluceth-20, menthyl lactate, propylene glycol, ethylhexylglycerin, PPG-12/SMDI copolymer, disodium EDTA, beta-glucan (357-178)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CORTISONE 10 INTENSIVE MOISTURE CREME
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PETROLATUM (UNII: 4T6H12BN9U) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) CETYL ALCOHOL (UNII: 936JST6JCN) ALOE VERA LEAF (UNII: ZY81Z83H0X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) CHAMOMILE (UNII: FGL3685T2X) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) NIACINAMIDE (UNII: 25X51I8RD4) POLYSORBATE 60 (UNII: CAL22UVI4M) STEARAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: W6000VEI5Y) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) METHYL GLUCETH-20 (UNII: J3QD0LD11P) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TRANSFORMING GROWTH FACTOR BETA RECEPTOR TYPE 3 (UNII: 18YWT2KYS8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0022-0 1 in 1 CARTON 01/01/2023 1 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:41167-0022-5 1 in 1 CARTON 01/01/2023 2 56 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:41167-0022-6 2 in 1 CARTON 01/01/2023 3 56 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2023 Labeler - Chattem, Inc. (003336013)