Label: PLEO FORM- formic acid solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 60681-0114-3 - Packager: Sanum Kehlbeck GmbH & Co. KG
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 23, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- Indications
- ACTIVE INGREDIENT
- Tamper Evident
- DOSAGE
- WARNING
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
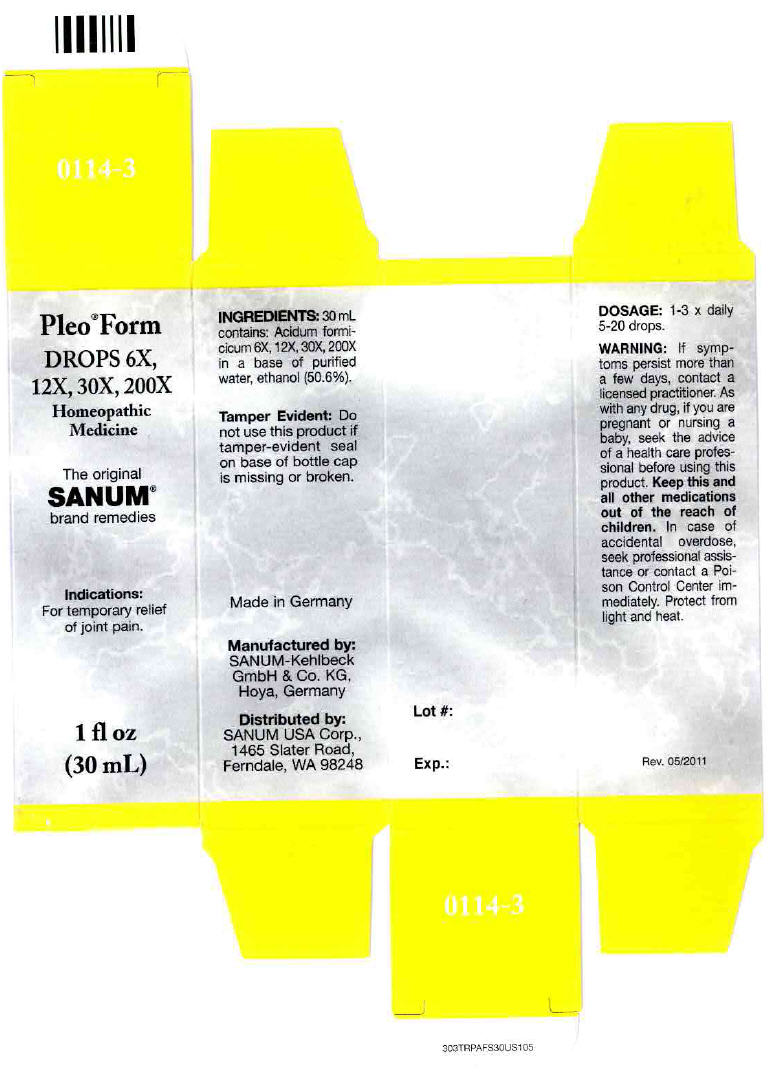
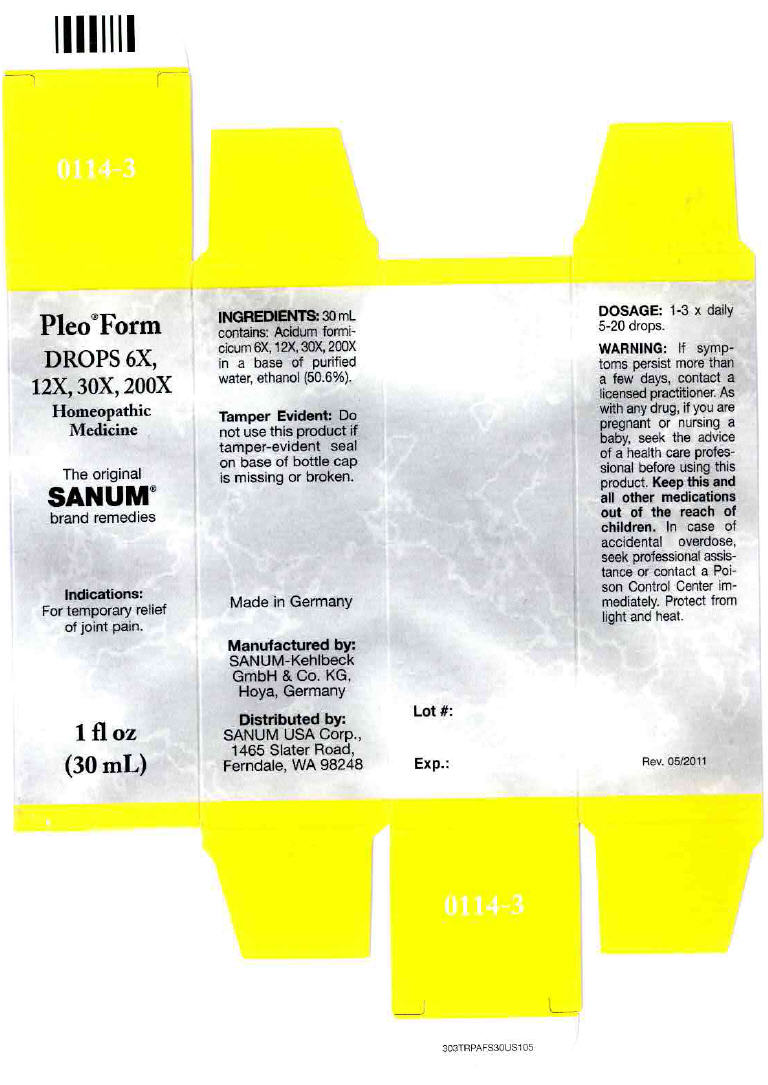
- PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
PLEO FORM
formic acid solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60681-0114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength formic acid (UNII: 0YIW783RG1) (formic acid - UNII:0YIW783RG1) formic acid 200 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60681-0114-3 1 in 1 CARTON 1 30 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 12/09/2004 Labeler - Sanum Kehlbeck GmbH & Co. KG (318386133)