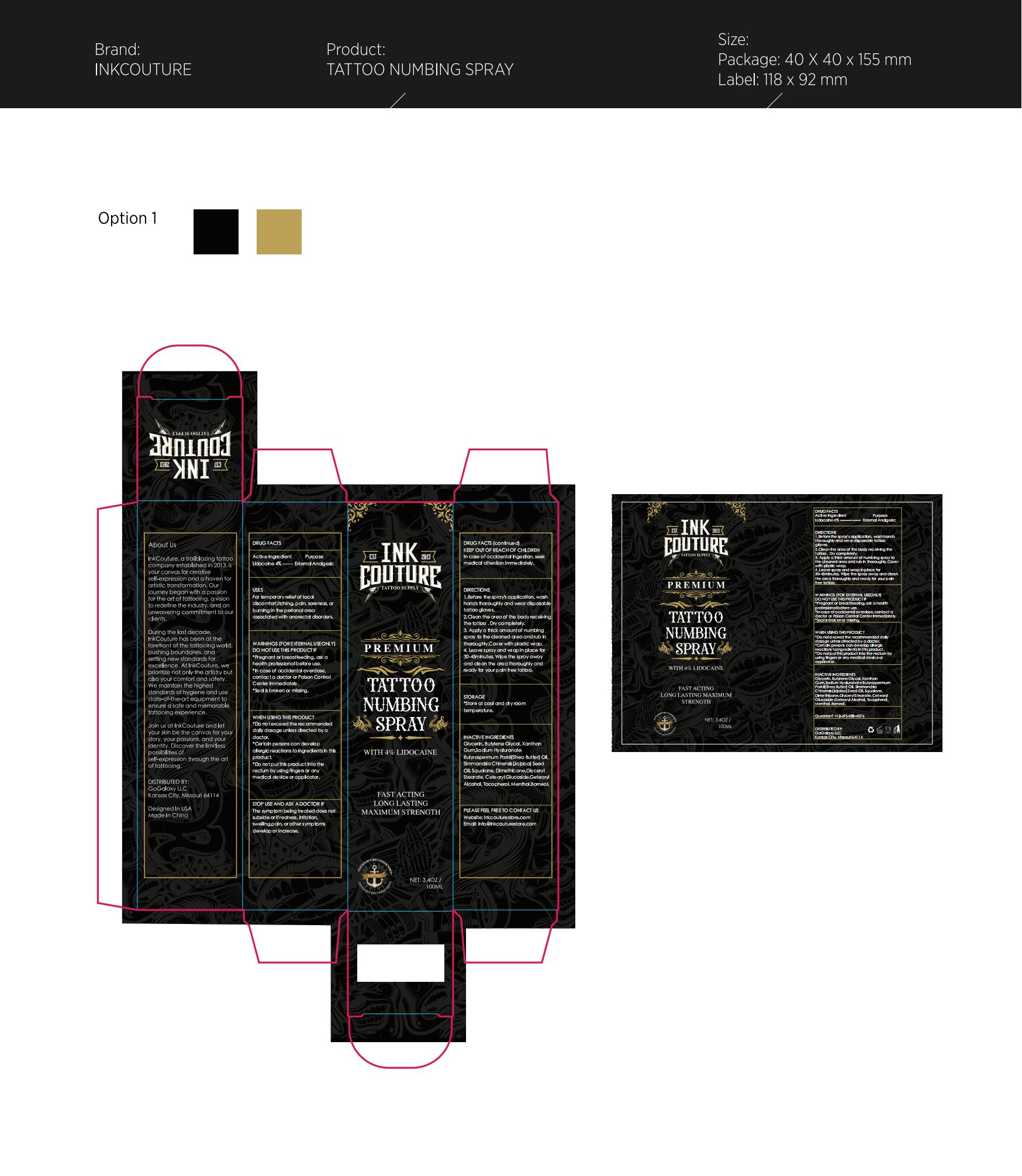
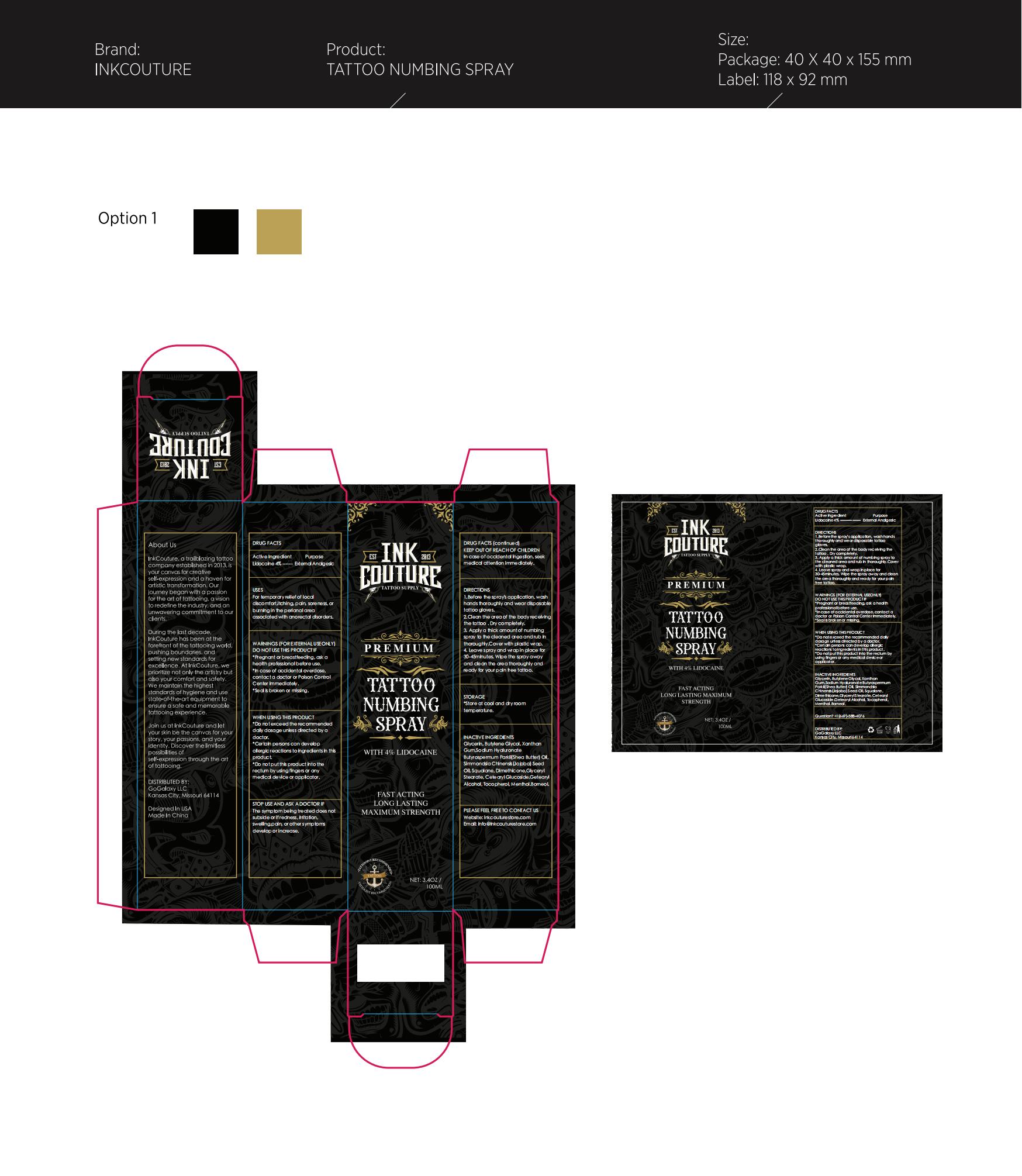
Label: INKCOUTURE TATTOO NUMBING .SPRAY- tattoo numbing spray liquid
- NDC Code(s): 83565-006-01
- Packager: Stellans Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- When Using
- Stop Use
- Keep Oot Of Reach Of Children
-
Directions
1.Before the spray's application, wash hands thoroughly and wear disposable tattoo gloves.
2.Clean the area of the body receiving the tattoo . Dry completely.
3. Apply a thick amount of numbing spray to the cleaned area and rub in thoroughly.Cover with plastic wrap.
4. Leave spray and wrap in place for 30-45minutes. Wipe the spray away and clean the area thoroughly and ready for your pain free tattoo. - Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INKCOUTURE TATTOO NUMBING .SPRAY
tattoo numbing spray liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83565-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 10 g in 100 mL Inactive Ingredients Ingredient Name Strength JOJOBA OIL (UNII: 724GKU717M) DIMETHICONE (UNII: 92RU3N3Y1O) MENTHOL (UNII: L7T10EIP3A) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SQUALANE (UNII: GW89575KF9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BORNEOL (UNII: M89NIB437X) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL 1-STEARATE (UNII: 258491E1RZ) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83565-006-01 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/01/2023 Labeler - Stellans Inc. (111157321) Establishment Name Address ID/FEI Business Operations Stellans Inc. 111157321 label(83565-006) , manufacture(83565-006)