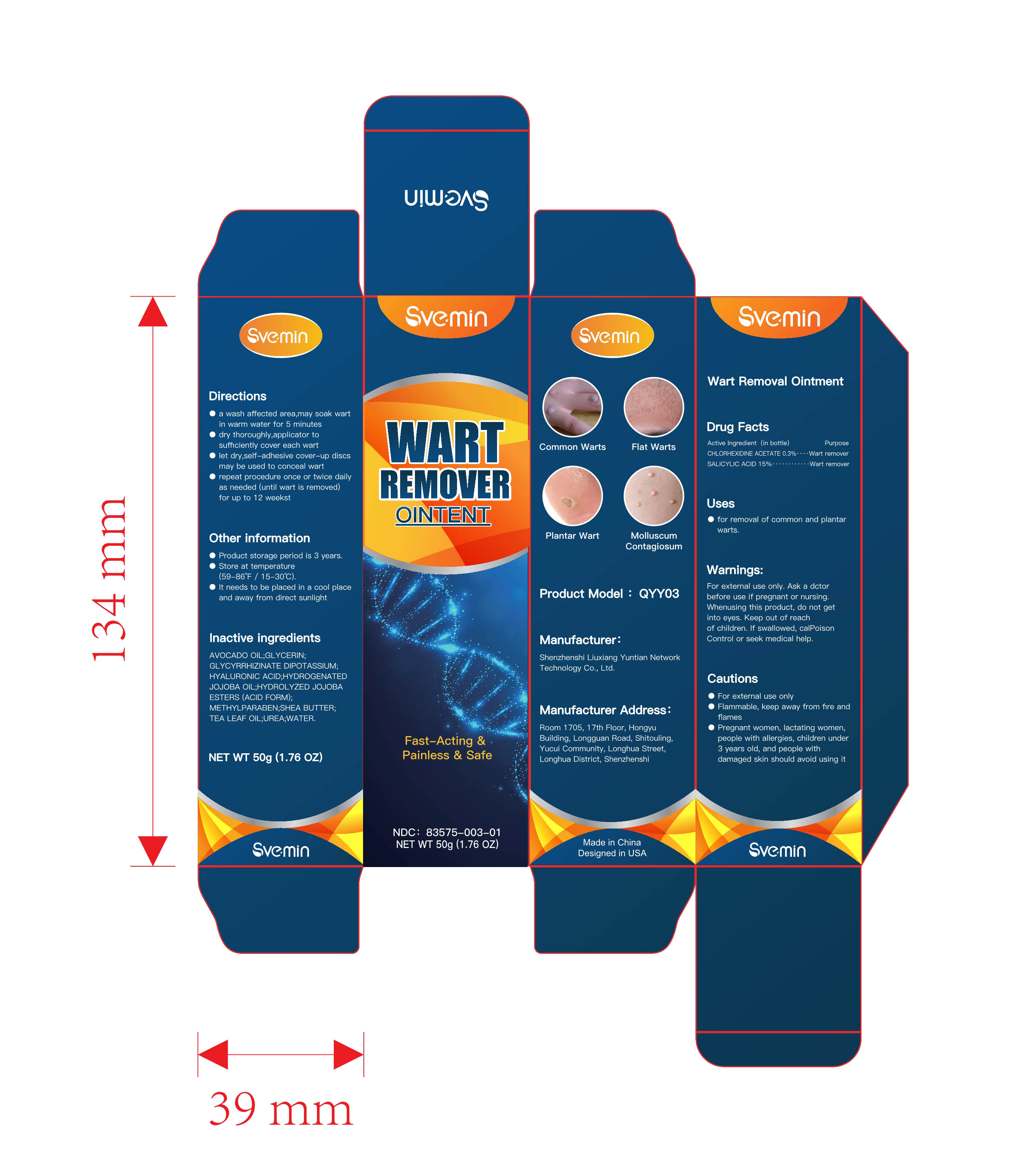
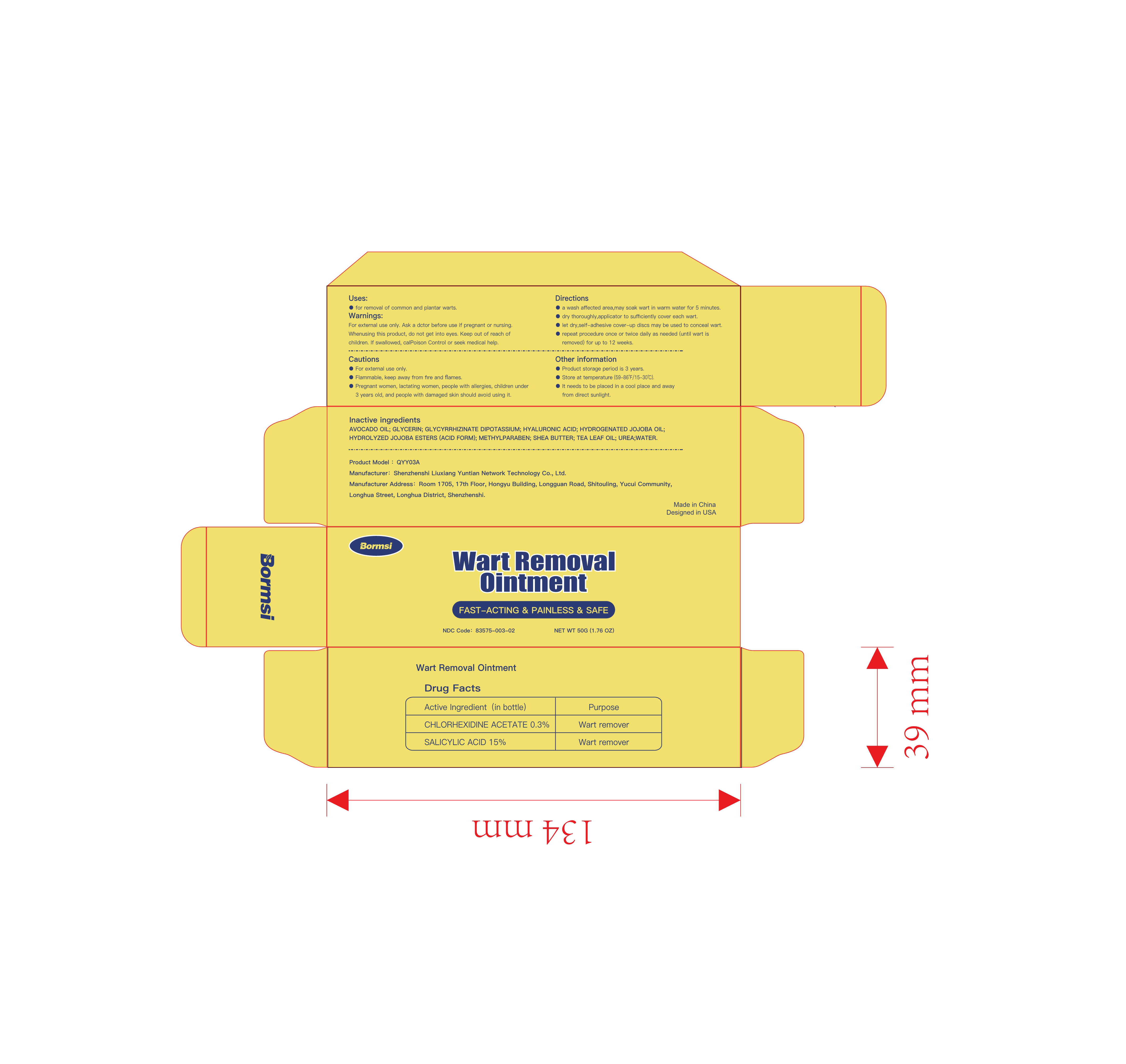
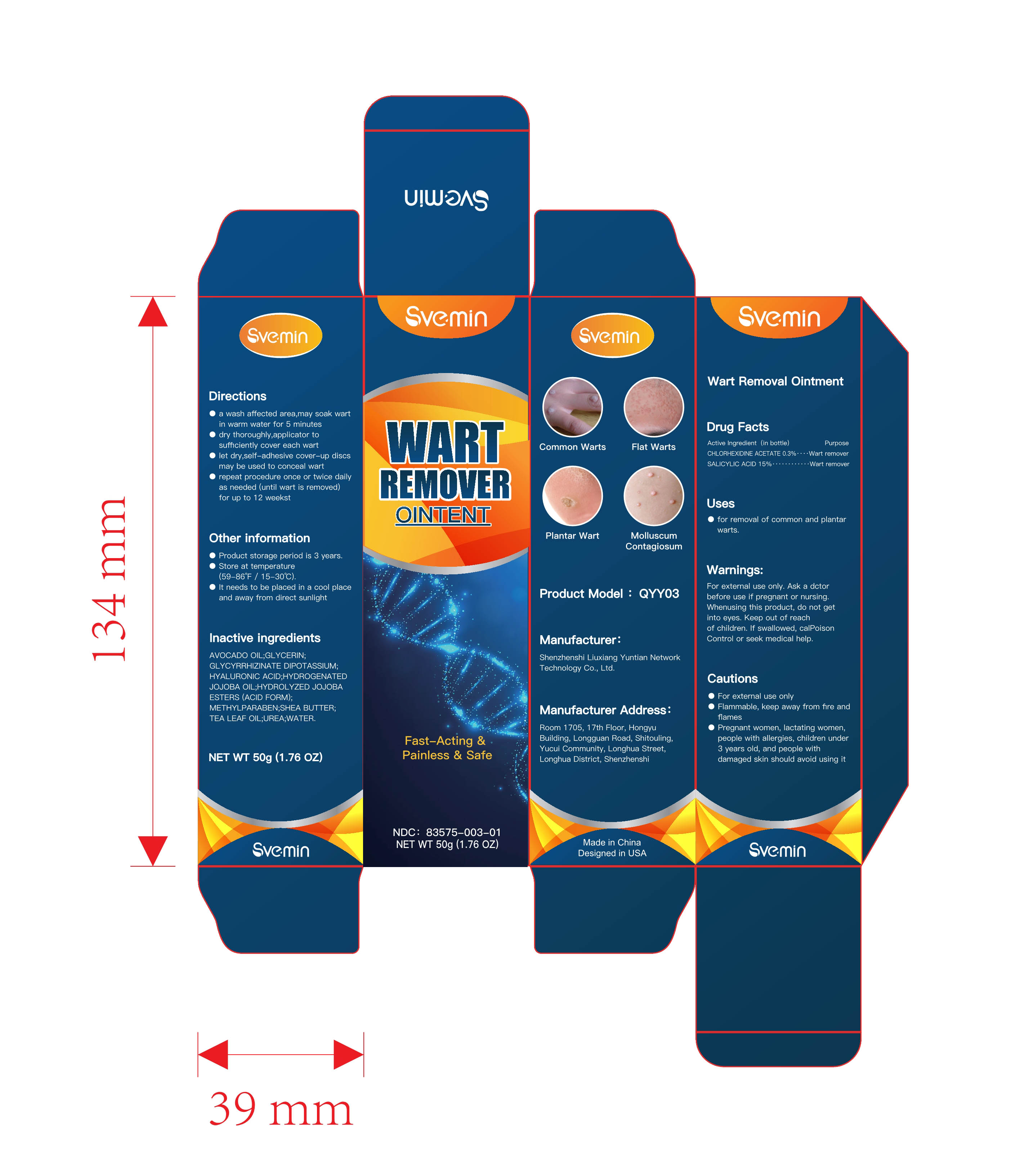
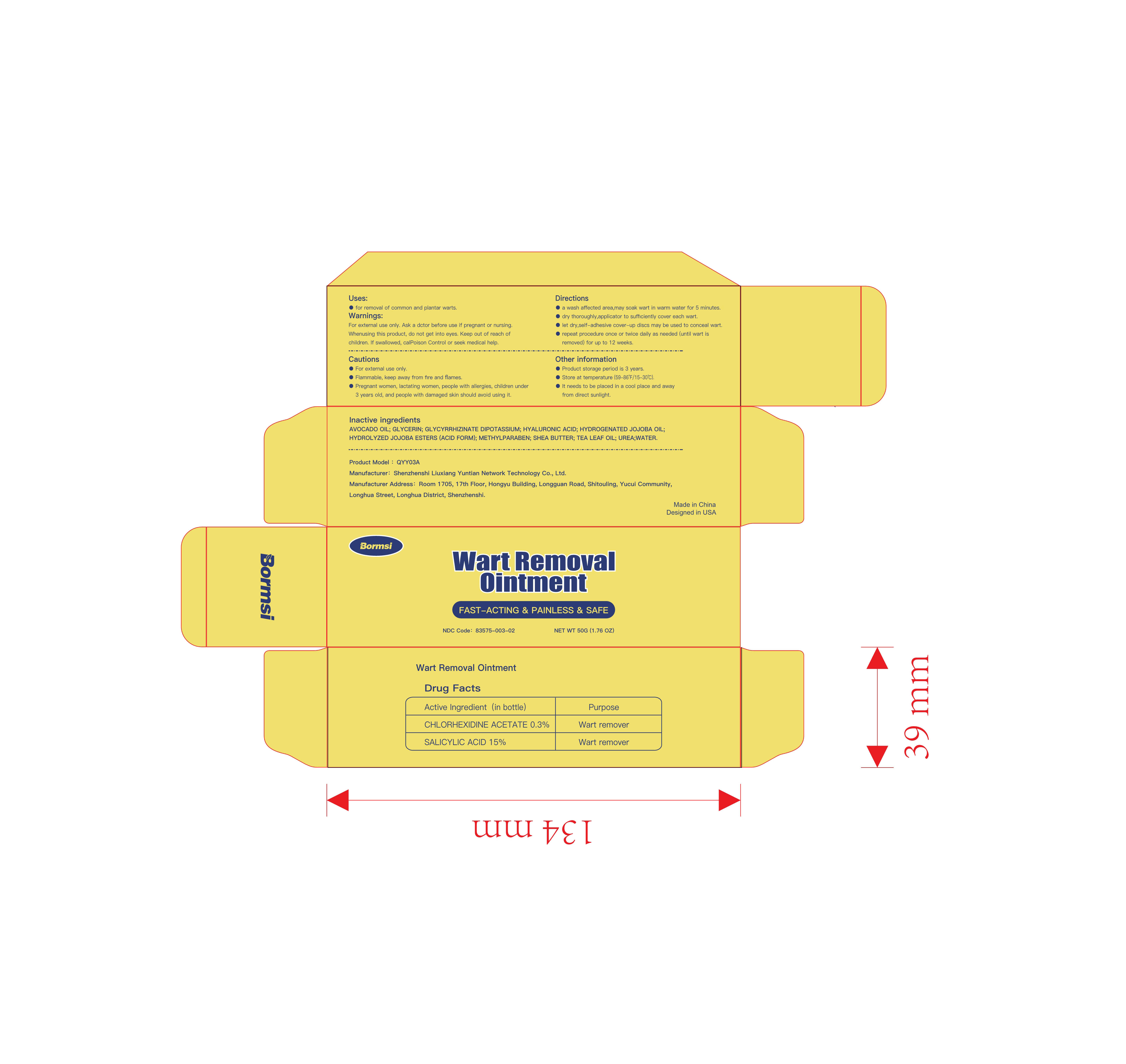
Label: WART REMOVAL ointment
- NDC Code(s): 83575-003-01, 83575-003-02
- Packager: Shenzhenshi Liuxiang Yuntian Network Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- DO NOT USE
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- a wash affected area , may soak wart in warmwater for 5 minuteso
- dry thoroughly, apply one drop at a time withapplicator to sufficiently cover each wart
- let dry , self-adhesive cover-up discs may beused to conceal wart
- Repeat procedure once or twice daily as needed (until wart is removed) for up to 12 weeks - Other information
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WART REMOVAL
wart removal ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83575-003 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 15 g in 100 g CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE 0.3 g in 100 g Inactive Ingredients Ingredient Name Strength SHEA BUTTER (UNII: K49155WL9Y) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) UREA (UNII: 8W8T17847W) HYALURONIC ACID (UNII: S270N0TRQY) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) AVOCADO OIL (UNII: 6VNO72PFC1) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) HYDROGENATED JOJOBA OIL (UNII: 7F674YQ5SO) METHYLPARABEN (UNII: A2I8C7HI9T) TEA LEAF OIL (UNII: VC855RRT77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83575-003-01 50 g in 1 BOX; Type 0: Not a Combination Product 10/30/2023 2 NDC:83575-003-02 50 g in 1 BOX; Type 0: Not a Combination Product 10/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 10/30/2023 Labeler - Shenzhenshi Liuxiang Yuntian Network Technology Co., Ltd. (713044573) Establishment Name Address ID/FEI Business Operations Shenzhenshi Liuxiang Yuntian Network Technology Co., Ltd. 713044573 manufacture(83575-003)