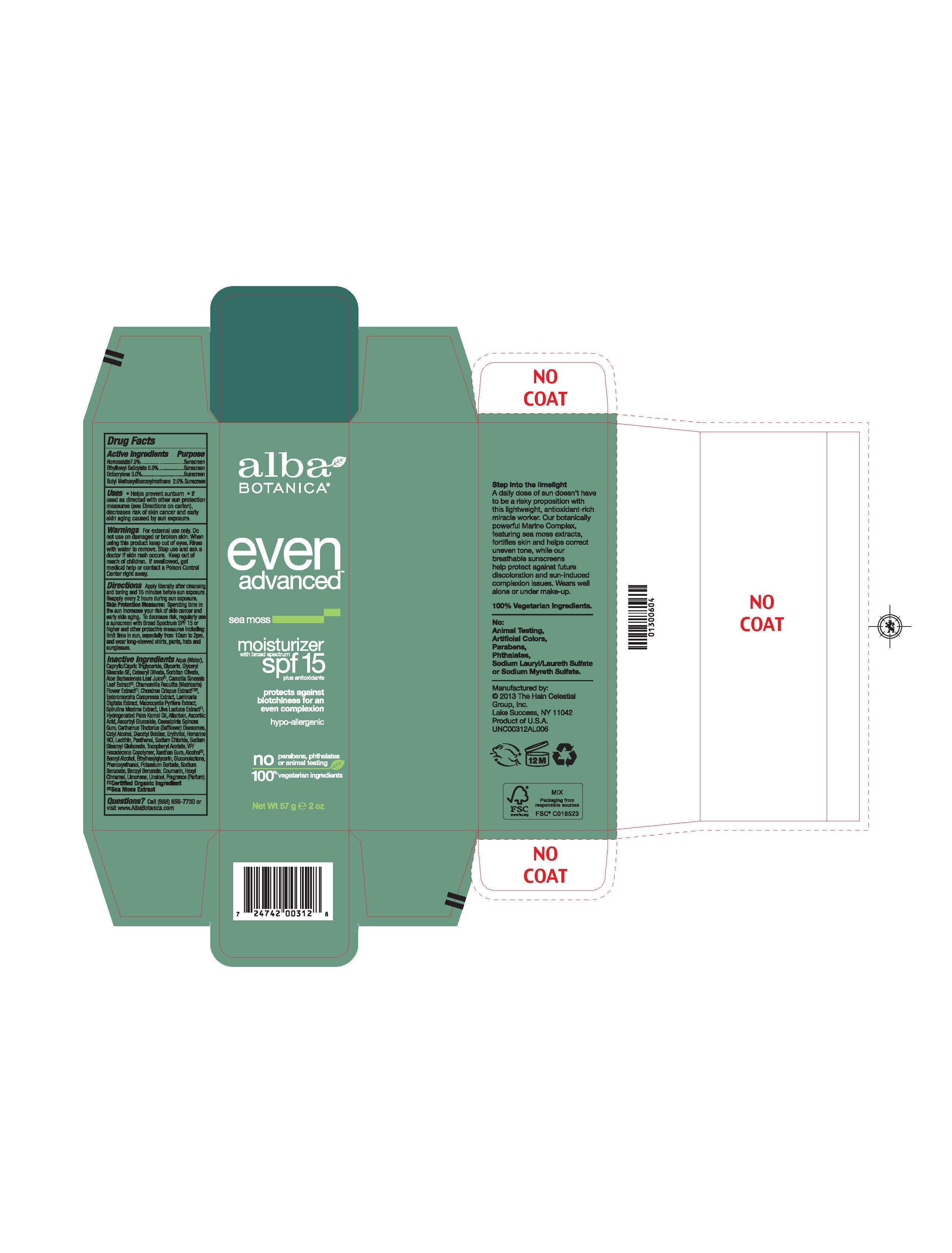
Label: ALBA EVEN ADVANCED SEA MOSS MOISTURIZER SPF15- homosalate, ethylhexyl salicylate, octocrylene, butyl methoxydibenzoylmethane lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 61995-2031-2 - Packager: The Hain Celestial Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Water, Glycerin, Glyceryl Stearate SE, Cetyl Alcohol, VP/Hexadecene Copolymer, Caprylic/Capric Triglyceride, Hydrogenated Palm Cernel Oil, Aloe Barbadensis Leaf Juice(1), Camellia Sinensis Leaf Extract (1), Chamomilla Recutita (Matricaria) Flower Extract (1), Chondrus Crispus Extract (1), (2), Enteromorpha Compressa Extract, Laminaria Digitata Extract, Macrocystis Pyrifera Extract, Spirulina Maxima Extract, Ulva Lactuca Extract (1), Allantoin, Ascorbic Acid, Ascorbyl Glucoside, Caesalpinia Spinosa Gum, Carthamus Tinctorius (Safflower) Oleosomes, Cetearyl Olivate, , Lecithin, Diacetyl Boldine, Erythritol, Homarine HCL, Panthenol, Gluconolactone, Sodium Chloride, Sodium Stearoyl Glutamate, Sorbitan Olivate, Tocopheryl Acetat, Xanthan Gum, Alcohol (1), Benzyl Alcohol, Ethylhexylglycerin, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate.
(1) Certified Organicf Ingredient, (2) Sea Moss Extract
-
INDICATIONS & USAGE
Helps prevents sunburns. If used as directed with other sun protection measures, decreases risk of skin cancer and early skin aging caused by sun exposure. Skin Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease risk, regularly use sunscreen with Broad Spectrum SPF 15 or higher and other protective measures including: limit time in sun, especially from10am to 2pm, and wear long sleeved shirts, pants, hats and sunglases.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALBA EVEN ADVANCED SEA MOSS MOISTURIZER SPF15
homosalate, ethylhexyl salicylate, octocrylene, butyl methoxydibenzoylmethane lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61995-2031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALCOHOL (UNII: 3K9958V90M) ULVA LACTUCA (UNII: PHR3P25W6Y) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) VINYLPYRROLIDONE/HEXADECENE COPOLYMER (UNII: KFR5QEN0N9) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CETYL ALCOHOL (UNII: 936JST6JCN) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHAMOMILE (UNII: FGL3685T2X) XANTHAN GUM (UNII: TTV12P4NEE) HYDROGENATED PALM KERNEL OIL (UNII: FM8D1RE2VP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAESALPINIA SPINOSA RESIN (UNII: WL3883U2PO) CETEARYL OLIVATE (UNII: 58B69Q84JO) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITAN OLIVATE (UNII: MDL271E3GR) SODIUM BENZOATE (UNII: OJ245FE5EU) HOMARINE HYDROCHLORIDE (UNII: 8866LNG61N) CHONDRUS CRISPUS CARRAGEENAN (UNII: UE856F2T78) ULVA COMPRESSA (UNII: SXZ209FM33) LAMINARIA DIGITATA (UNII: 15E7C67EE8) MACROCYSTIS PYRIFERA (UNII: K31S3OG5C4) SPIRULINA MAXIMA (UNII: 9K7IG15M0Q) ALLANTOIN (UNII: 344S277G0Z) ASCORBIC ACID (UNII: PQ6CK8PD0R) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) DIACETYL BOLDINE (UNII: 37727Z7M0I) ERYTHRITOL (UNII: RA96B954X6) PANTHENOL (UNII: WV9CM0O67Z) GLUCONOLACTONE (UNII: WQ29KQ9POT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61995-2031-2 1 in 1 CARTON 01/13/2014 1 113 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/13/2014 Labeler - The Hain Celestial Group, Inc. (117115556) Registrant - The Hain Celestial Group, Inc. (081512382) Establishment Name Address ID/FEI Business Operations The Hain Celestial Group, Inc. 081512382 manufacture(61995-2031)