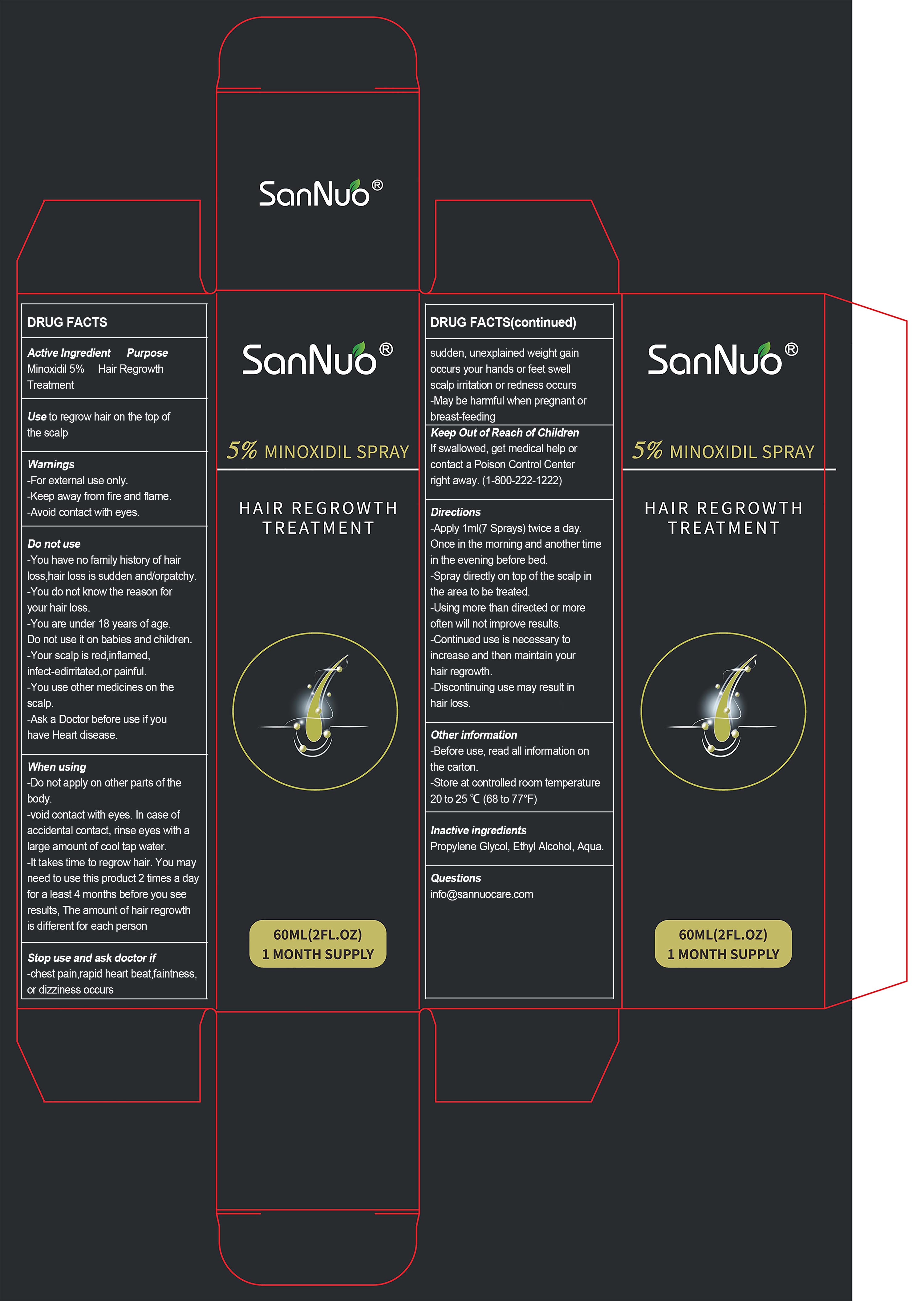
Label: 5% MINOXIDIL HAIR REGROWTH TREATMENT- sannuo 5% minoxidil hair regrowth treatment spray
- NDC Code(s): 83648-002-01
- Packager: Shenzhen Baixingchen Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- ASK DOCTOR
- WARNINGS
-
DO NOT USE
Do not use
-You have no family history of hair loss,hair loss is sudden and/orpatchy.
-You do not know the reason for your hair loss.
-You are under 18 years of age.
Do not use it on babies and children.
-Your scalp is red,inflamed, infect-edirritated,or painful.
-You use other medicines on the scalp. -
WHEN USING
When using
-Do not apply on other parts of the body.
-void contact with eyes. In case of accidental contact, rinse eyes with a large amount of cool tap water.
-It takes time to regrow hair. You may need to use this product 2 times a day for a least 4 months before you see results, The amount of hair regrowth is different for each person - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- OTHER SAFETY INFORMATION
-
DOSAGE & ADMINISTRATION
Directions
-Apply 1ml (7 Sprays) twice a day.
Once in the morning and another time in the evening before bed.
-Spray directly on top of the scalp in the area to be treated.
-Using more than directed or more often will not improve results.
-Continued use is necessary to increase and then maintain your hair regrowth.
-Discontinuing use may result in hair loss. - INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
5% MINOXIDIL HAIR REGROWTH TREATMENT
sannuo 5% minoxidil hair regrowth treatment sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83648-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83648-002-01 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/28/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075357 10/28/2023 Labeler - Shenzhen Baixingchen Technology Co., Ltd. (411899311) Establishment Name Address ID/FEI Business Operations Shenzhen Baixingchen Technology Co., Ltd. 411899311 manufacture(83648-002)