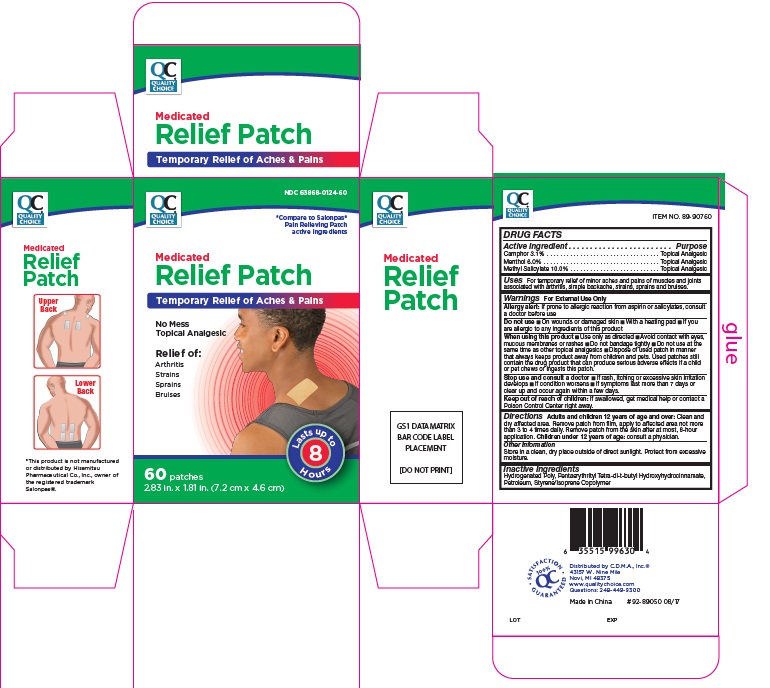
Label: MEDICATED PAIN RELIEF PATCHES- camphor, menthol, methyl salicylate patch
- NDC Code(s): 63868-124-20, 63868-124-60
- Packager: QUALITY CHOICE (Chain Drug Marketing Association)
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 14, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- For External Use Only
- Do not use:
-
When using this product
- Use only as directed
- Avoid contact with eyes, mucous membranes or rashes
- Do not bandage tighly
- Do not use at the same time as other topical analgesics
- Dispose of used patch in manner that keeps product away from childrens and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
- Stop use and consult a doctor
- Keep out of reach of children
- Uses
-
Directions
Adults and children 12 years of age and over: Clean and dry affected area. Remove patch from film, apply to affected area not more than 3 to 4 times daily. Remove patch from the skin after at most, 8-hour application.
Children under 12 years of age: consult physician.
- Questions or Comments
- Pain Relief Patch Label
-
INGREDIENTS AND APPEARANCE
MEDICATED PAIN RELIEF PATCHES
camphor, menthol, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-124 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.1 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) Product Characteristics Color Score Shape SQUARE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-124-60 60 in 1 BOX 09/01/2017 1 9 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:63868-124-20 20 in 1 BOX 09/01/2017 2 9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/01/2017 Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(63868-124)