Label: POWDER- titanium dioxide powder
- NDC Code(s): 61354-119-01, 61354-119-02
- Packager: Oxygen Development LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using
- Keep out of reach of children
- Directions
- Other Information
-
Inactive Ingredient
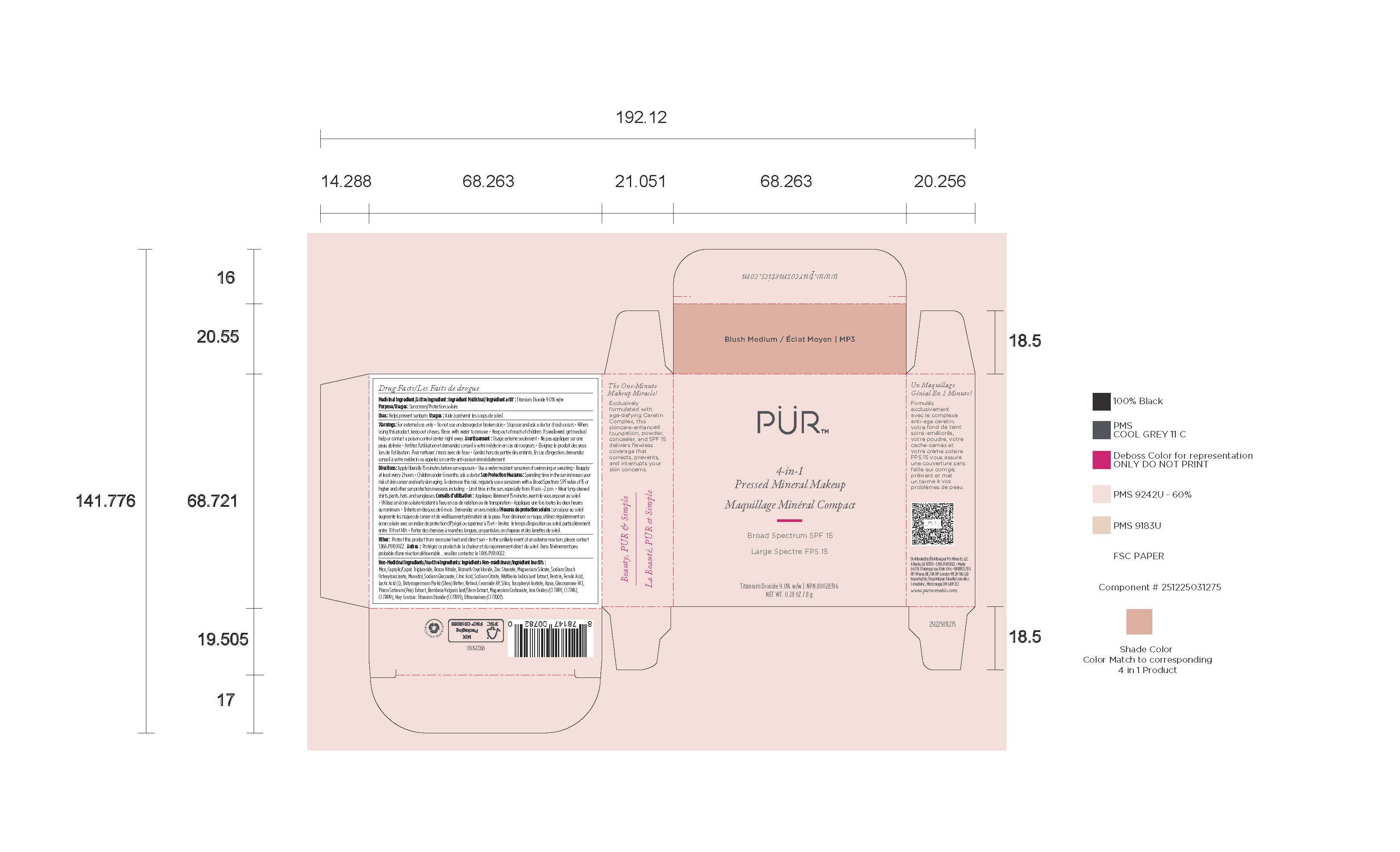
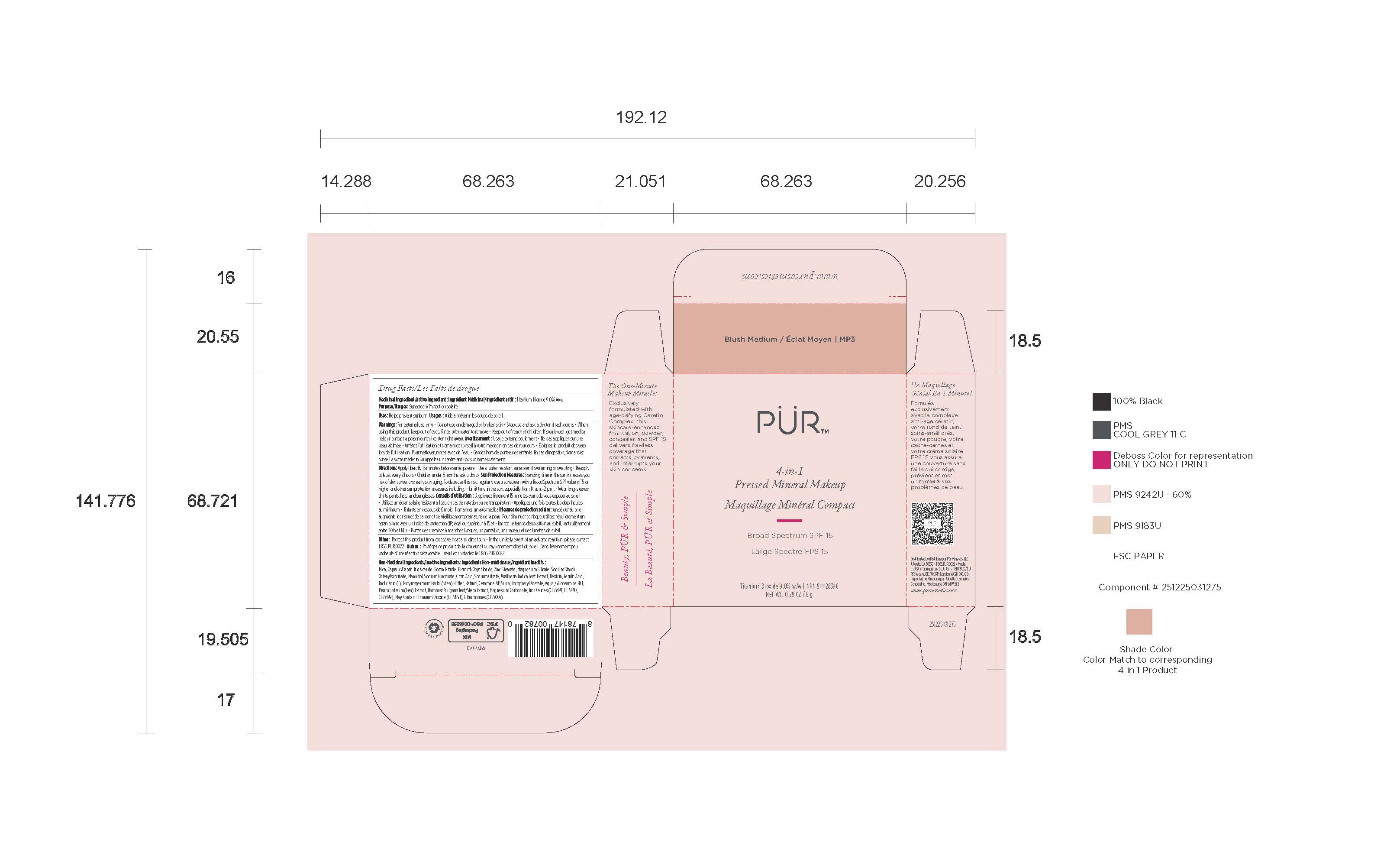
Mica, Caprylic/Capric Triglyceride, Boron Nitride, Bismuth Oxychloride, Zinc Stearate, Magnesium Silicate, Sodium Starch Octenylsuccinate, Mannitol, Sodium Gluconate, Citric Acid, Sodium Citrate, Waltheria Indica Leaf Extract, Dextrin, Ferulic Acid, Lactic Acid (L), Butyrospermum Parkii (Shea) Butter, Retinol, Ceramide AP, Silica, Tocopheryl Acetate, Aqua, Glucosamine HCI, Pisum Sativum (Pea) Extract, Bambusa Vulgaris Leaf/Stem Extract, Magnesium Carbonate, Iron Oxides (CI 77491, CI 77492, CI 77499), May Contain: Titanium Dioxide (CI 77891), Ultramarines (CI 77007).
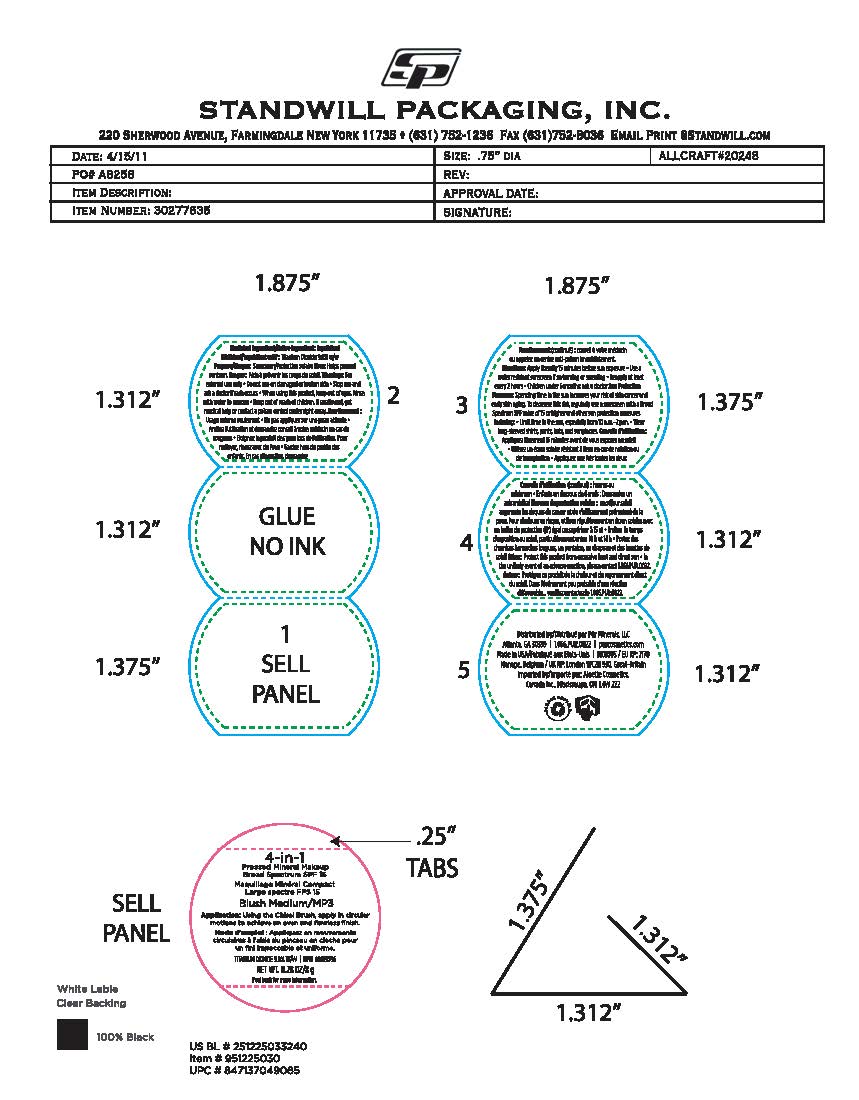
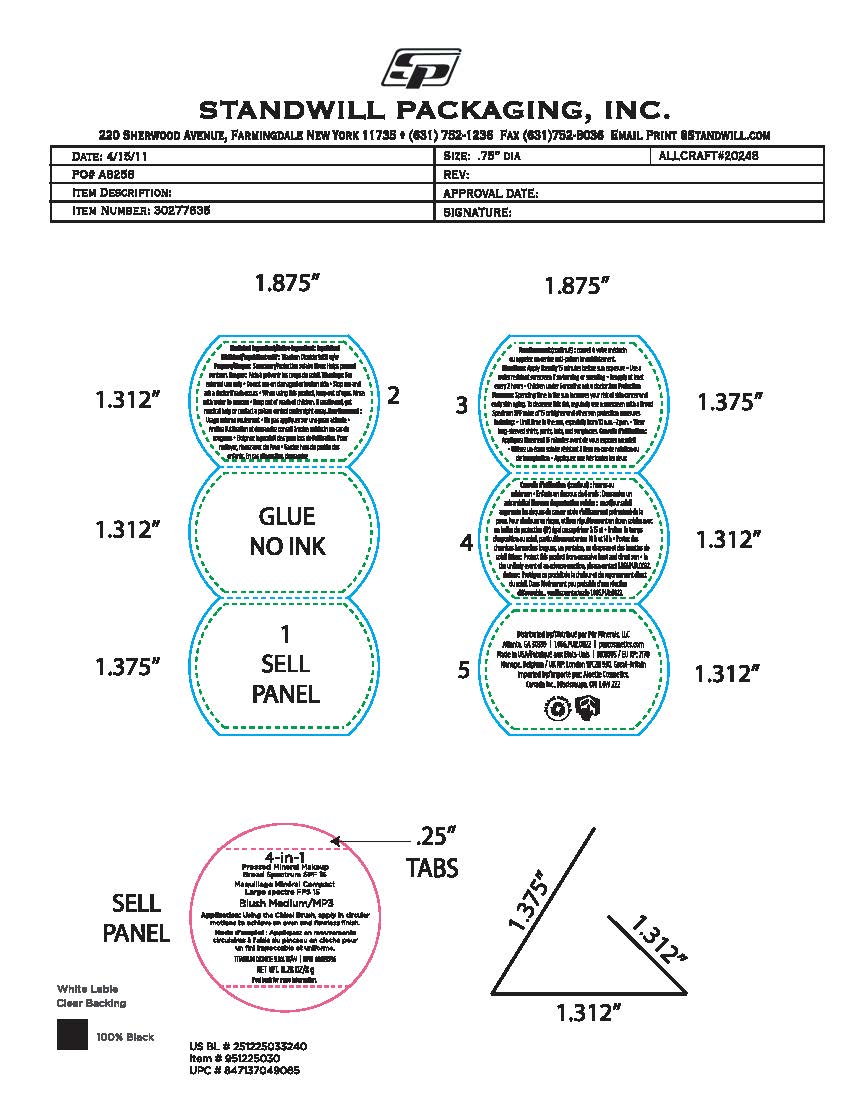
- Package label - Principal display panel
-
INGREDIENTS AND APPEARANCE
POWDER
titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61354-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERRIC OXIDE RED (UNII: 1K09F3G675) (FERRIC OXIDE RED - UNII:1K09F3G675) FERRIC OXIDE RED 1.75 mg in 100 mg TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10 mg in 100 mg Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) 8 mg in 100 mg MAGNESIUM SILICATE (UNII: 9B9691B2N9) 2 mg in 100 mg MANNITOL (UNII: 3OWL53L36A) 1.67 mg in 100 mg BORON NITRIDE (UNII: 2U4T60A6YD) 8 mg in 100 mg SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 0.5 mg in 100 mg SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) 1.89 mg in 100 mg MICA (UNII: V8A1AW0880) 47.33 mg in 100 mg BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) 10 mg in 100 mg ZINC STEARATE (UNII: H92E6QA4FV) 2.8 mg in 100 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61354-119-02 1 in 1 CARTON 10/27/2023 1 NDC:61354-119-01 100 mg in 1 CUP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/27/2023 Labeler - Oxygen Development LLC (137098492) Establishment Name Address ID/FEI Business Operations Oxygen Development LLC 137098492 manufacture(61354-119)