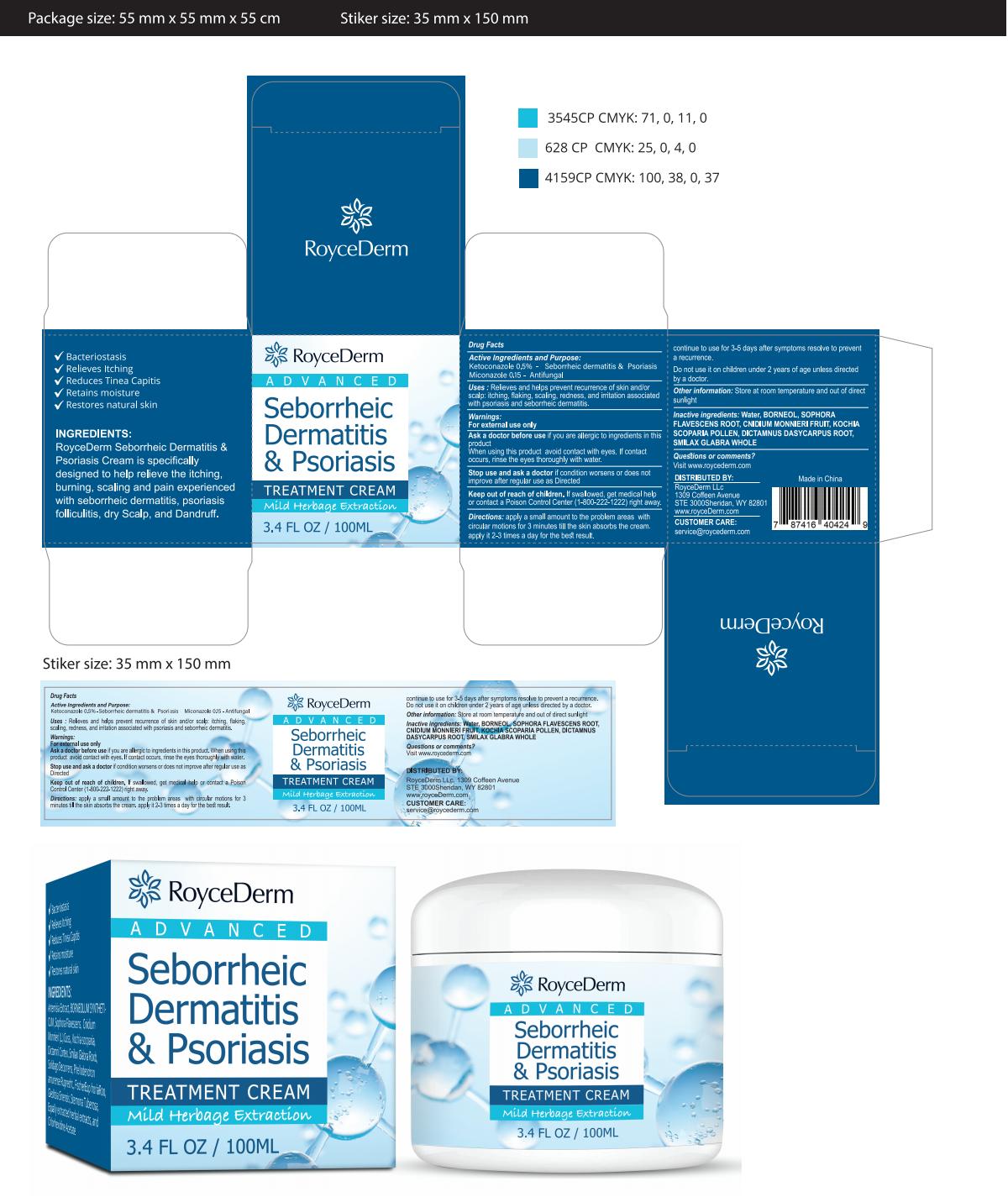
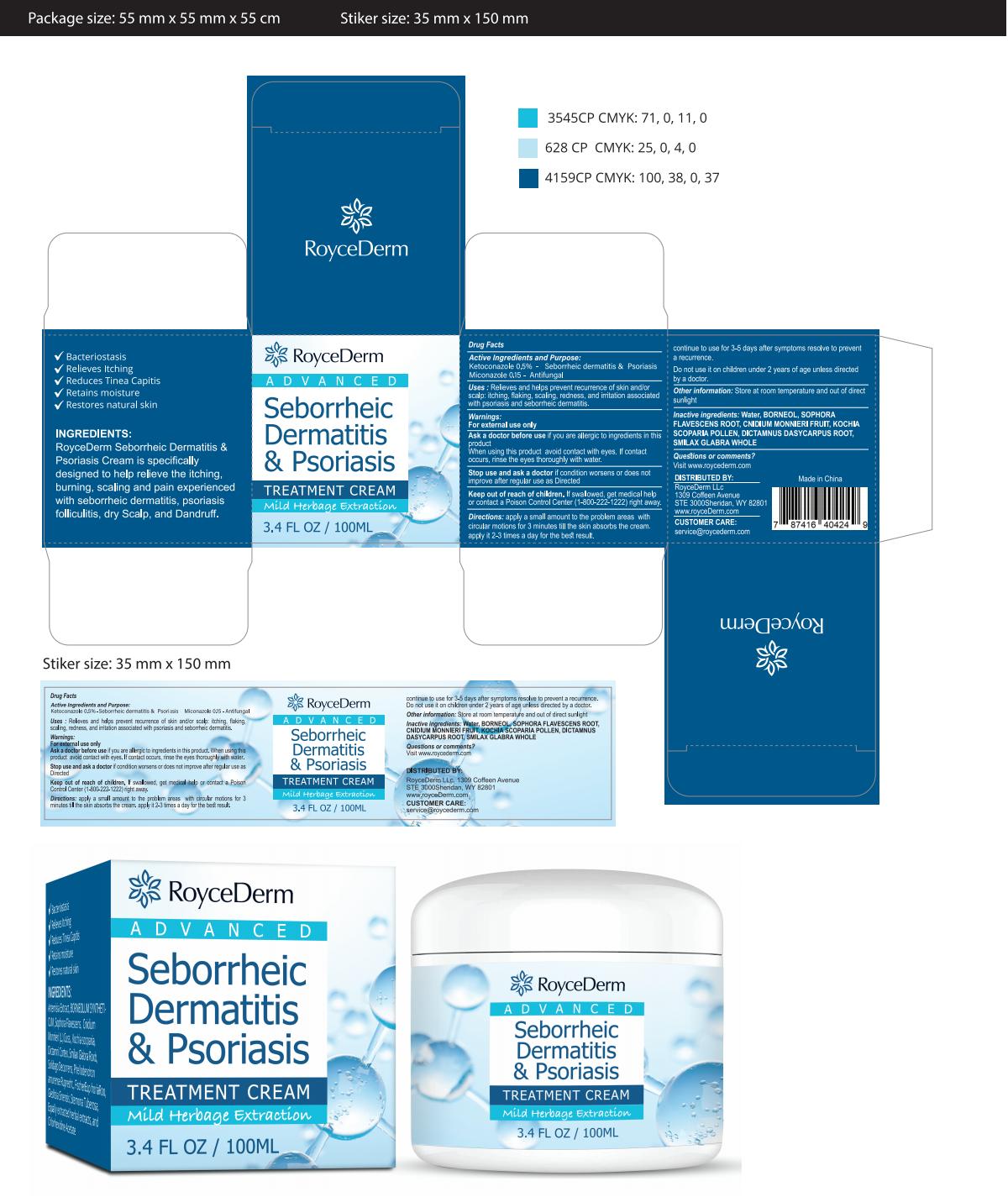
Label: ROYCEDERM SEBORRHEIC DERMATITIS PSORIASIS- dermatitis cream cream
- NDC Code(s): 83771-001-01
- Packager: Inner Mongolia Green source pharmaceutical Products Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
apply a small amount to the problem areas withcircular motions for 3 minutes till the skin absorbs the cream.apply it 2-3 times a day for the best result
continue to use for 3-5 days after symptoms resolve to preventa recurrence
Do not use it on children under 2 years of age unless directedby a doctor. - Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROYCEDERM SEBORRHEIC DERMATITIS PSORIASIS
dermatitis cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83771-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE (UNII: 7NNO0D7S5M) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE 0.15 g in 100 mL KETOCONAZOLE (UNII: R9400W927I) (KETOCONAZOLE - UNII:R9400W927I) KETOCONAZOLE 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ELYMUS VIOLACEUS WHOLE (UNII: 05VL37FC3P) GANODERMA LUCIDUM STEM (UNII: U8PA41532G) SOPHORA FLAVESCENS WHOLE (UNII: X8KX602M5L) NYCTANTHES ARBOR-TRISTIS WHOLE (UNII: FM5DVE2OJ1) MINT (UNII: FV98Z8GITP) EUPHORBIA HIRTA (UNII: L13YF113GN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83771-001-01 100 mL in 1 CANISTER; Type 0: Not a Combination Product 10/26/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 10/26/2023 Labeler - Inner Mongolia Green source pharmaceutical Products Co., LTD (699508923) Establishment Name Address ID/FEI Business Operations Inner Mongolia Green source pharmaceutical Products Co., LTD 699508923 label(83771-001) , manufacture(83771-001)