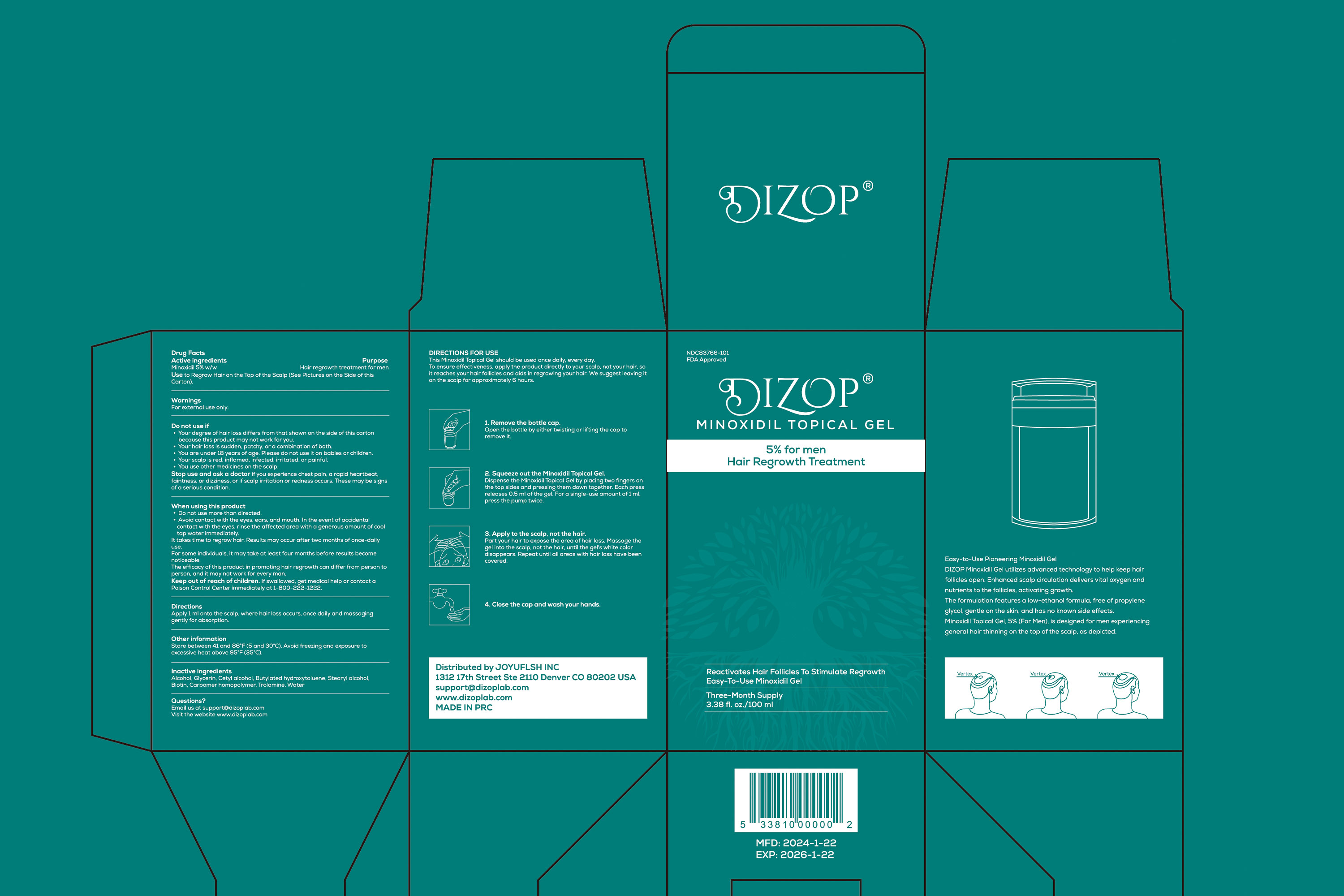
Label: DIZOP MINOXIDIL TOPICAL GEL- minoxidil 5% gel
- NDC Code(s): 83766-101-01, 83766-101-02
- Packager: Shenzhen Joyuflsh Technology Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DO NOT USE
Your degree of hair loss differs from that shown on the side of this carton because this product may not work for you.
Your hair loss is sudden, patchy, or a combination of both.
You are under 18 years of age. Please do not use it on babies or children.
Your scalp is red, inflamed, infected, irritated, or painful.
You use other medicines on the scalp. -
WHEN USING
Do not use more than directed.
Avoid contact with the eyes, ears, and mouth. In the event of accidental contact withthe eyes, rinse the affected area with a generous amount of cool tap waterimmediately.
lt takes time to regrow hair. Results may occur after two months of once-daily useFor some individuals, it may take at least four months before results becomenoticeable.
The efficacy of this product in promoting hair regrowth can differ from person to person, and it may not work for every man. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIZOP MINOXIDIL TOPICAL GEL
minoxidil 5% gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83766-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STEARIC ACID (UNII: 4ELV7Z65AP) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) GLYCERIN (UNII: PDC6A3C0OX) OLEIC ACID (UNII: 2UMI9U37CP) WATER (UNII: 059QF0KO0R) BIOTIN (UNII: 6SO6U10H04) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83766-101-02 1 in 1 CARTON 10/26/2023 1 NDC:83766-101-01 100 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 10/26/2023 Labeler - Shenzhen Joyuflsh Technology Co.,Ltd. (603012375) Registrant - Shenzhen Joyuflsh Technology Co.,Ltd. (603012375) Establishment Name Address ID/FEI Business Operations Shenzhen Joyuflsh Technology Co.,Ltd. 603012375 manufacture(83766-101)