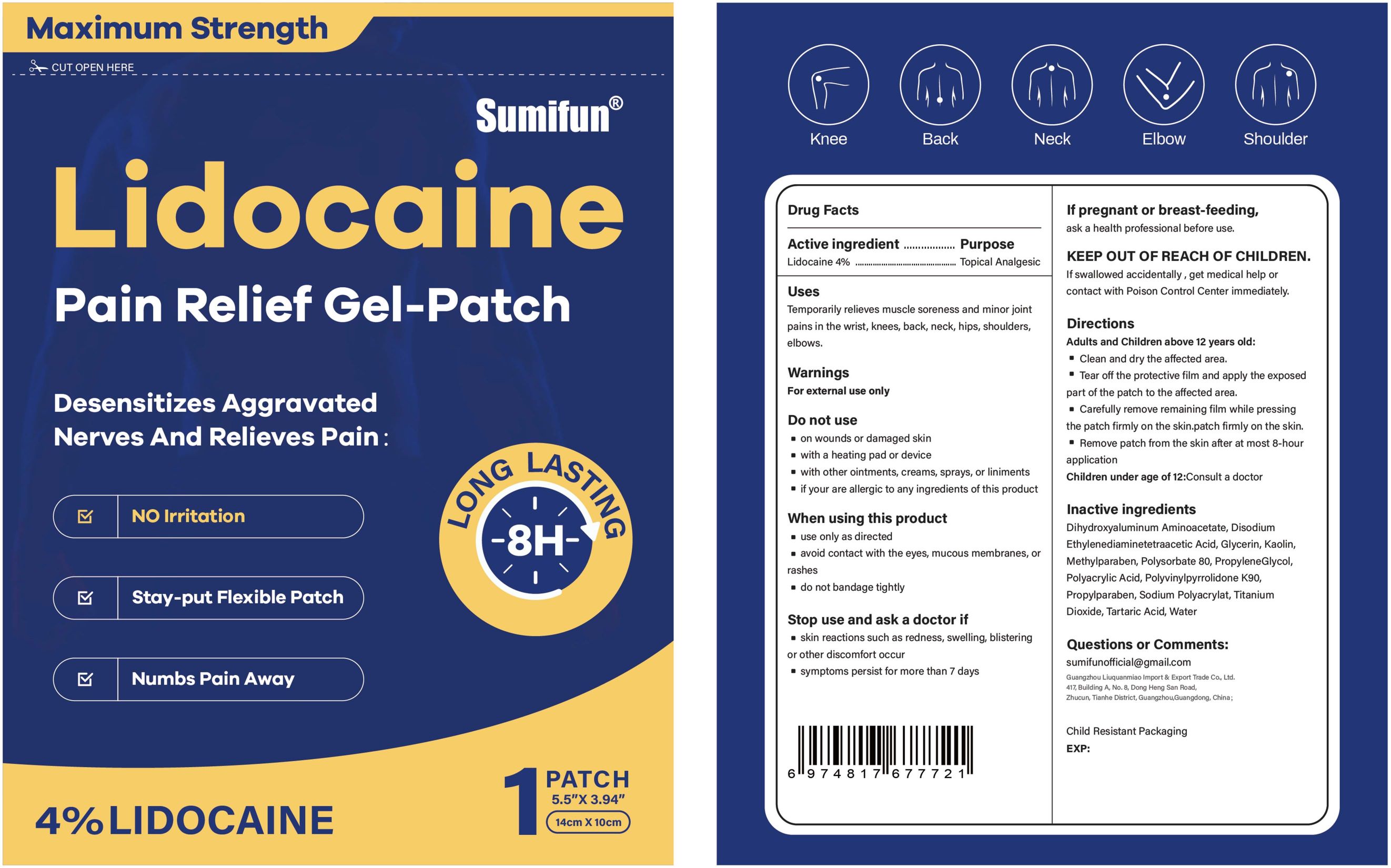
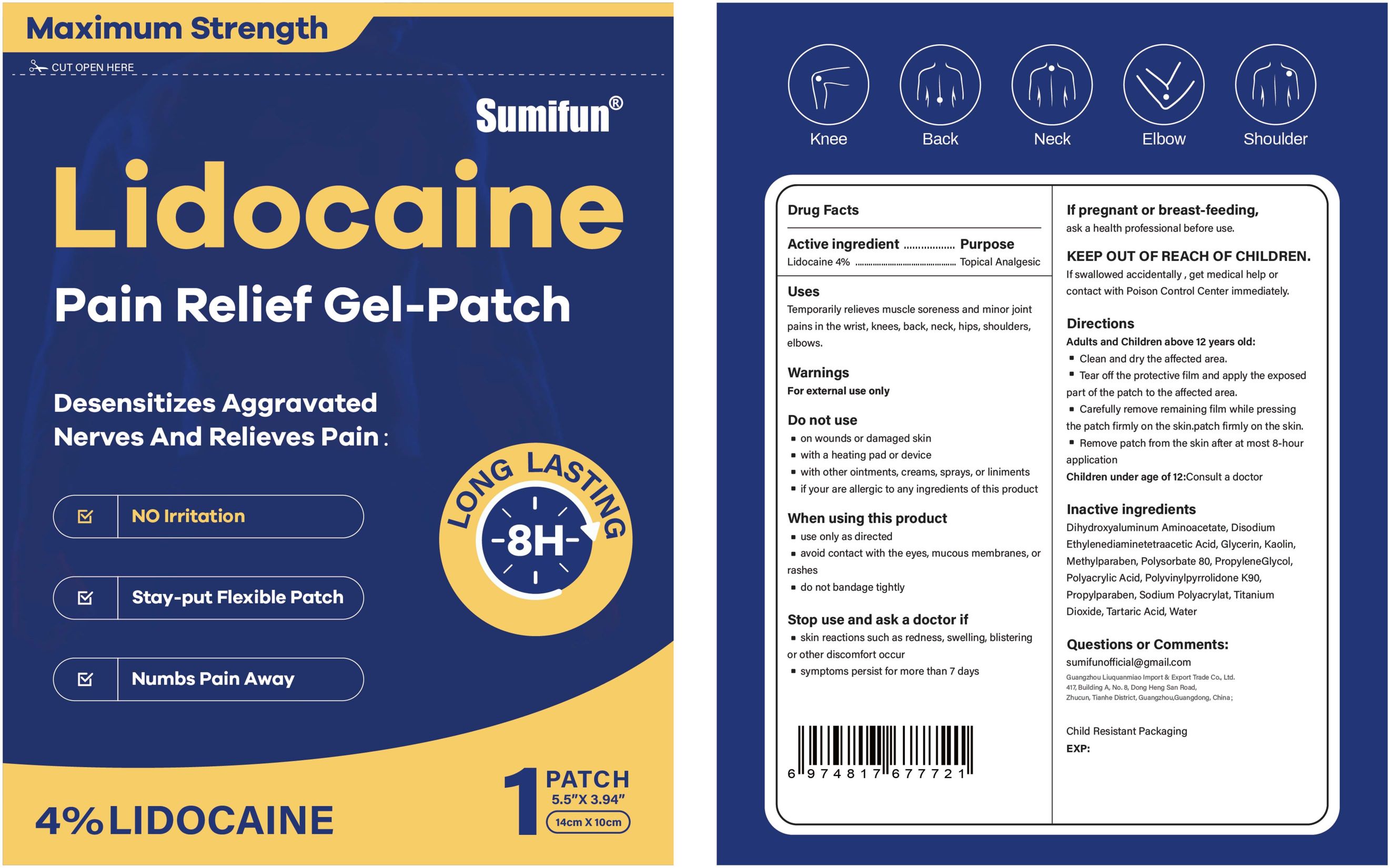
Label: LIDOCAINE PAIN RELIEF GEL-PATCH- lidocaine patch
- NDC Code(s): 83602-157-01, 83602-157-15, 83602-157-30
- Packager: Guangzhou Liuquanmiao Import & Export Trade Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use ... if
- ... ask a doctor if
- If pregnant or breast-feeding
- KEEP OUT OF REACH OF CHILDREN
-
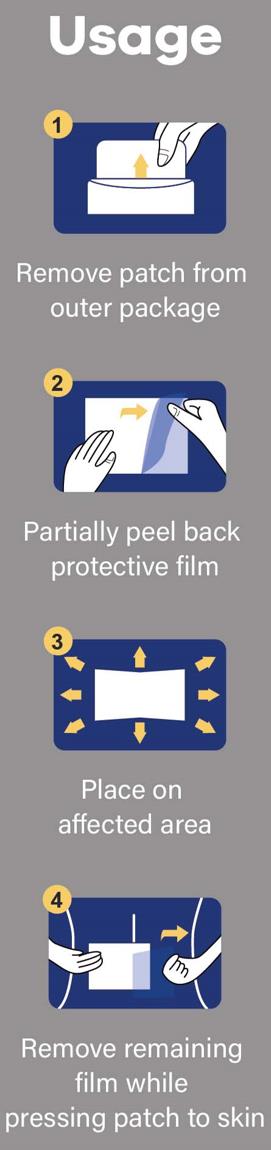
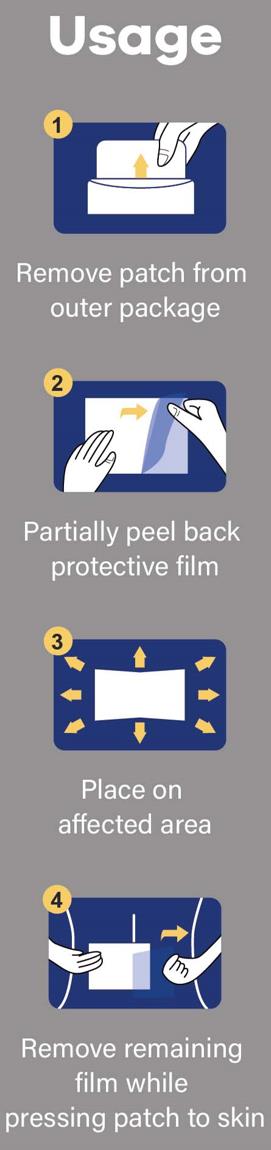
Directions
Adults and Children above 12 years old:
■ Clean and dry the affected area.
■ Tear off the proctive film and apply the exposed part of the patch to the affected area.
■ Carefully remove remaining film while pressing the patch firmly on the skin.
■ Remove patch from the skin after at most 8-hour application.
Children under age of 12: Consult a doctor
- Inactive ingredients
- Usage
- Questions or Comments
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCAINE PAIN RELIEF GEL-PATCH
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83602-157 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLYCERIN (UNII: PDC6A3C0OX) POVIDONE K90 (UNII: RDH86HJV5Z) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) KAOLIN (UNII: 24H4NWX5CO) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83602-157-15 15 in 1 BOX 10/27/2023 1 NDC:83602-157-01 12 g in 1 BAG; Type 0: Not a Combination Product 2 NDC:83602-157-30 30 in 1 BOX 02/05/2024 2 NDC:83602-157-01 12 g in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/27/2023 Labeler - Guangzhou Liuquanmiao Import & Export Trade Co., Ltd. (418399704) Registrant - Guangzhou Liuquanmiao Import & Export Trade Co., Ltd. (418399704) Establishment Name Address ID/FEI Business Operations Shanghai Chuangshi Medical Technology (Group) Co., Ltd. 546872672 manufacture(83602-157) , label(83602-157)