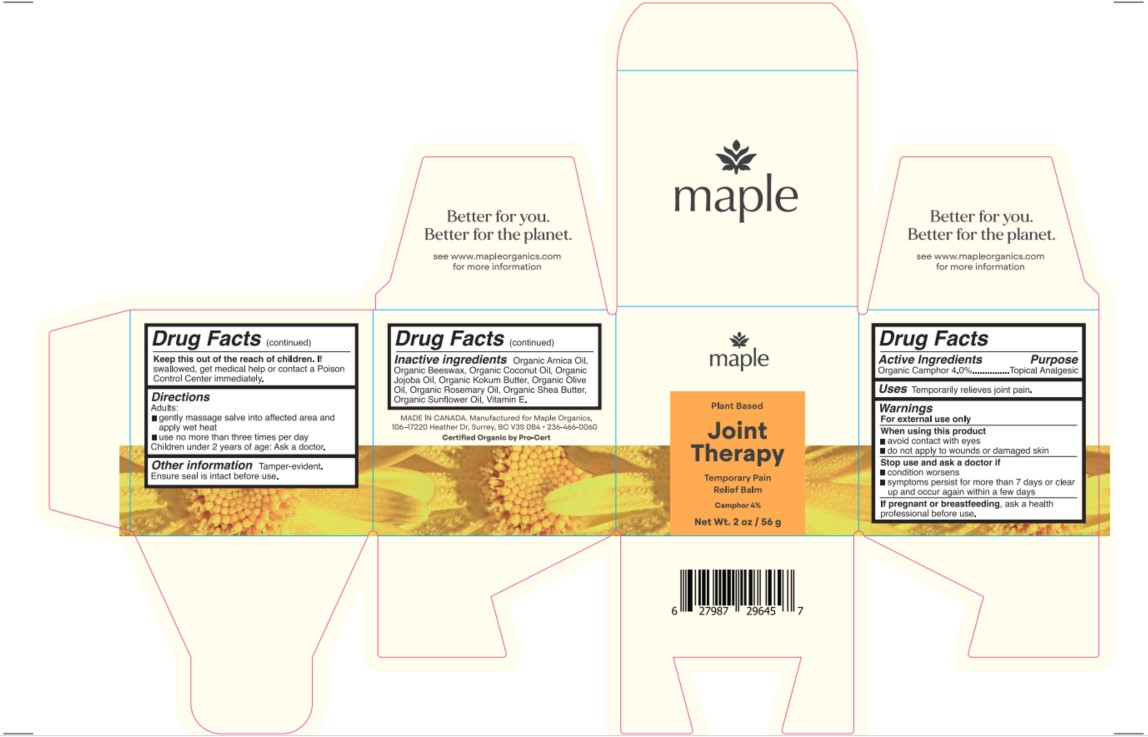
Label: JOINT THERAPY- camphor salve
- NDC Code(s): 83670-202-02
- Packager: Everlaan Organics Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JOINT THERAPY
camphor salveProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83670-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 4 g in 100 g Inactive Ingredients Ingredient Name Strength ROSEMARY OIL (UNII: 8LGU7VM393) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) ARNICA MONTANA (UNII: O80TY208ZW) JOJOBA OIL (UNII: 724GKU717M) GARCINIA INDICA SEED BUTTER (UNII: US2H3D7800) YELLOW WAX (UNII: 2ZA36H0S2V) COCONUT OIL (UNII: Q9L0O73W7L) SHEA BUTTER (UNII: K49155WL9Y) OLIVE OIL (UNII: 6UYK2W1W1E) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83670-202-02 1 in 1 CARTON 09/14/2023 1 56 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/14/2023 Labeler - Everlaan Organics Inc (243282059) Establishment Name Address ID/FEI Business Operations Everlaan Organics Inc 243282059 manufacture(83670-202)