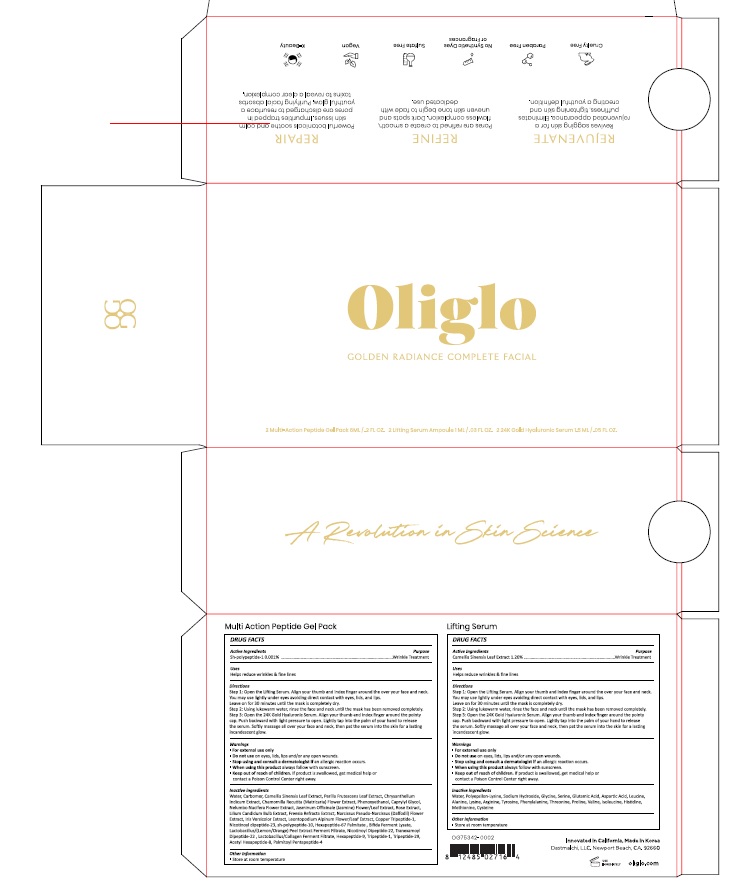
Label: OLIGLO GOLDEN RADIANCE COMPLETE FACIAL- sh-polypeptide-1, camellia sinensis leaf extract kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 73303-001-01, 73303-002-01, 73303-003-01 - Packager: Dastmalchi LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Oliglo Lifting Serum
Water, Polyepsilon-Lysine, Sodium Hydroxide, Glycine, Serine, Glutamic Acid, Aspartic Acid, Leucine, Alanine, Lysine, Arginine, Tyrosine, Phenylalanine, Threonine, Proline , Valine, Isoleucine, Histidine, Methionine, Cysteine
Oliglo Multi-Action Peptide Gel Pack
Water, Carbomer, Camellia Sinensis Leaf Extract, Perilla Frutescens Leaf Extract, Chrysanthellum Indicum Extract, Chamomilla Recutita (Matricaria) Flower Extract, Phenoxyethanol, Caprylyl Glycol, Nelumbo Nucifera Flower Extract, Jasminum Officinale (Jasmine) Flower/Leaf Extract, Rose Extract, Lilium Candidum Bulb Extract, Freesia Refracta Extract, Narcissus Pseudo-Narcissus (Daffodil) Flower Extract, Iris Versicolor Extract, Leontopodium Alpinum Flower/Leaf Extract, Copper Tripeptide-1, Nicotinoyl dipeptide-23, sh-polypeptide-10, Hexapeptide-67 Palmitate , Bifida Ferment Lysate, Lactobacillus/(Lemon/Orange) Peel Extract Ferment Filtrate, Nicotinoyl Dipeptide-22, Tranexamoyl Dipeptide-22 , Lactobacillus/Collagen Ferment Filtrate, Hexapeptide-9, Tripeptide-1, Tripeptide-29, Acetyl Hexapeptide-8, Palmitoyl Pentapeptide-4
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Step 1: Open the Lifting Serum. Align your thumb and index finger around the over your face and neck. You may use lightly under eyes avoding direct contact with eyes, lids, and lips. Leave on for 30 minutes until the mask is completely dry.
Step 2: Using lukewarm water, rinse the face and neck until the mask has been removed completely.
Setp 3: Open the 24K Gold Hyaluronic Serum. Align your thumb and index finger around the pointy cap. Push backward with light pressure to open. Lightly tap into the palm of your hand to release the serum. Softly massage all over your face and neck, then pat the serum into the skin for a lasting incandescent glow.
-
WARNINGS
For External Use Only.
Do not use on eyes, lids, lips and/or any open wounds.
Steop using and consult a dermatologist if an allergic reaction occurs.
When using this product always follow with sunscreen.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OLIGLO GOLDEN RADIANCE COMPLETE FACIAL
sh-polypeptide-1, camellia sinensis leaf extract kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73303-001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73303-001-01 1 in 1 CONTAINER; Type 0: Not a Combination Product 10/31/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKAGE 6 mL Part 2 1 VIAL 1 mL Part 1 of 2 OLIGLO MULTI-ACTION PEPTIDE GEL PACK
sh-polypeptide-1 liquidProduct Information Item Code (Source) NDC:73303-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BASIC FIBROBLAST GROWTH FACTOR (HUMAN) (UNII: S3529G9M9V) (BASIC FIBROBLAST GROWTH FACTOR (HUMAN) - UNII:S3529G9M9V) BASIC FIBROBLAST GROWTH FACTOR (HUMAN) 0.001 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) CHRYSANTHELLUM INDICUM TOP (UNII: STJ856D1Z0) CHAMOMILE (UNII: FGL3685T2X) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) NELUMBO NUCIFERA FLOWER (UNII: 61W322NLDV) JASMINUM OFFICINALE FLOWER (UNII: 0Q8K841432) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) NARCISSUS PSEUDONARCISSUS FLOWER (UNII: L879RBF1WN) LEONTOPODIUM NIVALE SUBSP. ALPINUM FLOWERING TOP (UNII: QQC1AK06RK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73303-002-01 6 mL in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/31/2019 Part 2 of 2 OLIGLO LIFTING SERUM
camellia sinensis leaf extract liquidProduct Information Item Code (Source) NDC:73303-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) (GREEN TEA LEAF - UNII:W2ZU1RY8B0) GREEN TEA LEAF 1.2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYEPSILON-LYSINE (4000 MW) (UNII: WB0M8X4TWR) SODIUM HYDROXIDE (UNII: 55X04QC32I) GLYCINE (UNII: TE7660XO1C) SERINE (UNII: 452VLY9402) GLUTAMIC ACID (UNII: 3KX376GY7L) ASPARTIC ACID (UNII: 30KYC7MIAI) LEUCINE (UNII: GMW67QNF9C) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73303-003-01 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/31/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/30/2019 Labeler - Dastmalchi LLC (079373461) Registrant - Dastmalchi LLC (079373461) Establishment Name Address ID/FEI Business Operations Picobio Co., Ltd. 688821818 manufacture(73303-001)