Label: BURN CREAM- benzalkonium chloride, lidocaine hci cream
- NDC Code(s): 69396-136-10, 69396-136-25
- Packager: Trifecta Pharmaceuticals USA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions
- Storage Information
- Keep out of reach of children
- Other Information
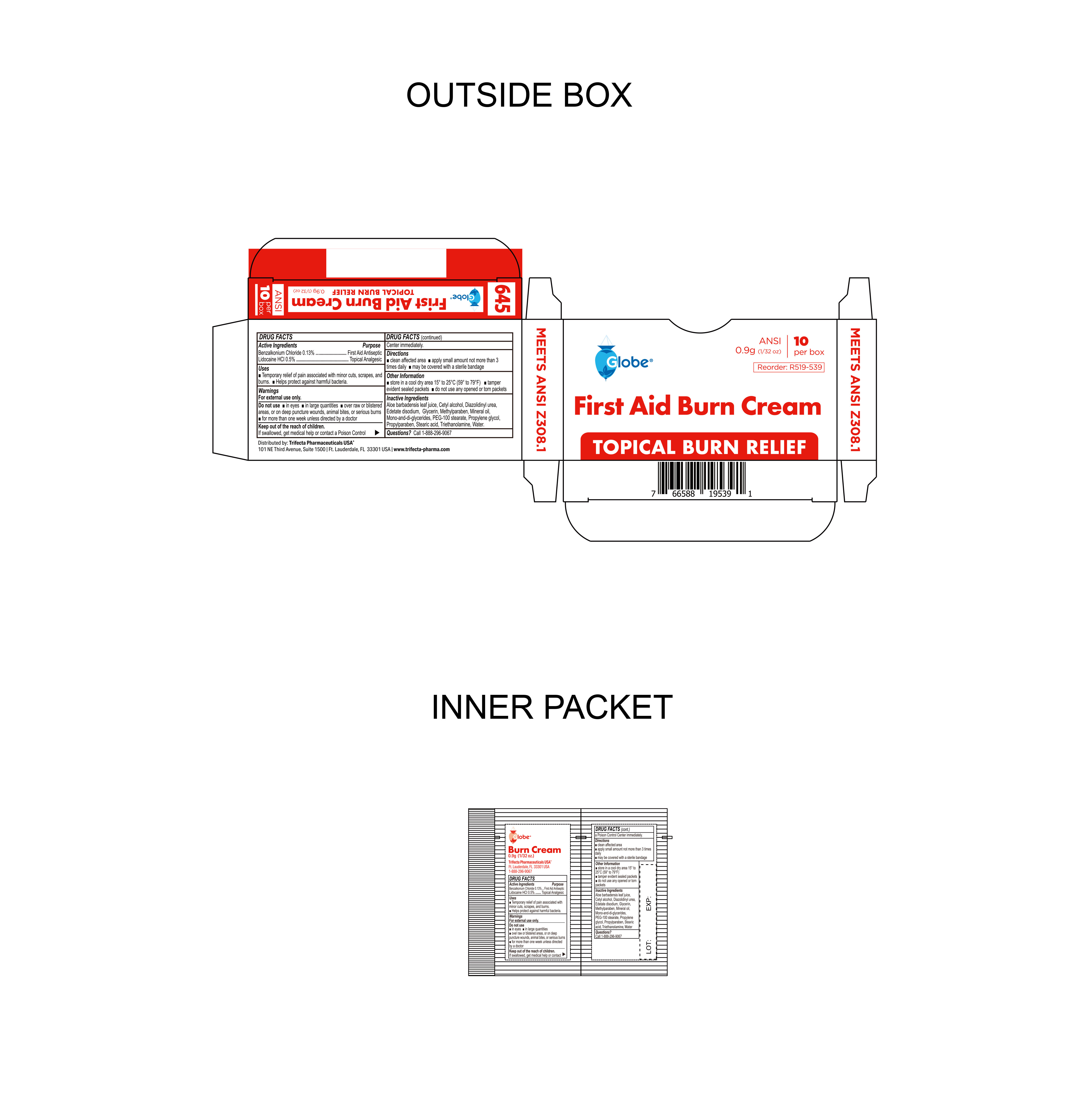
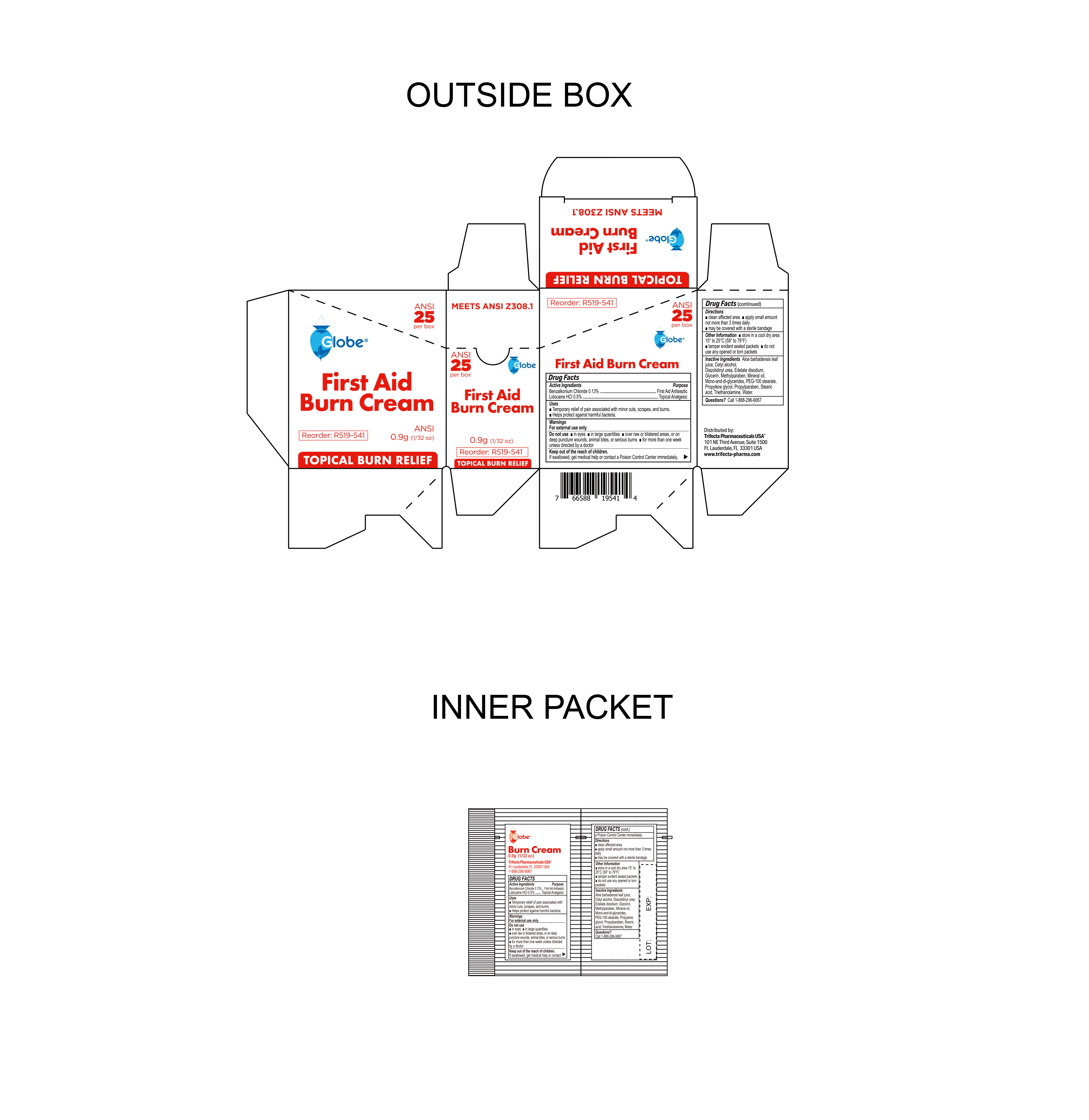
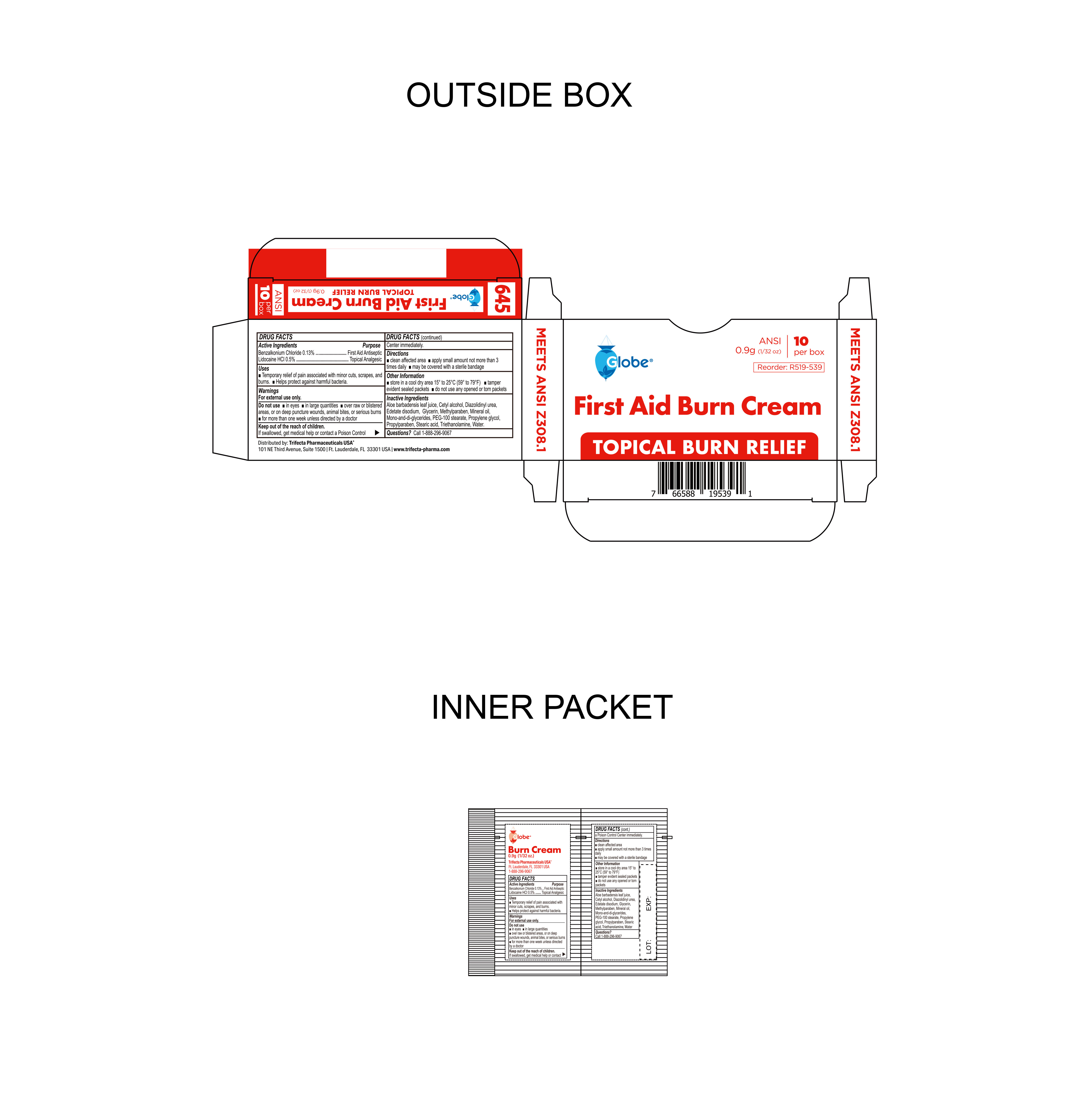
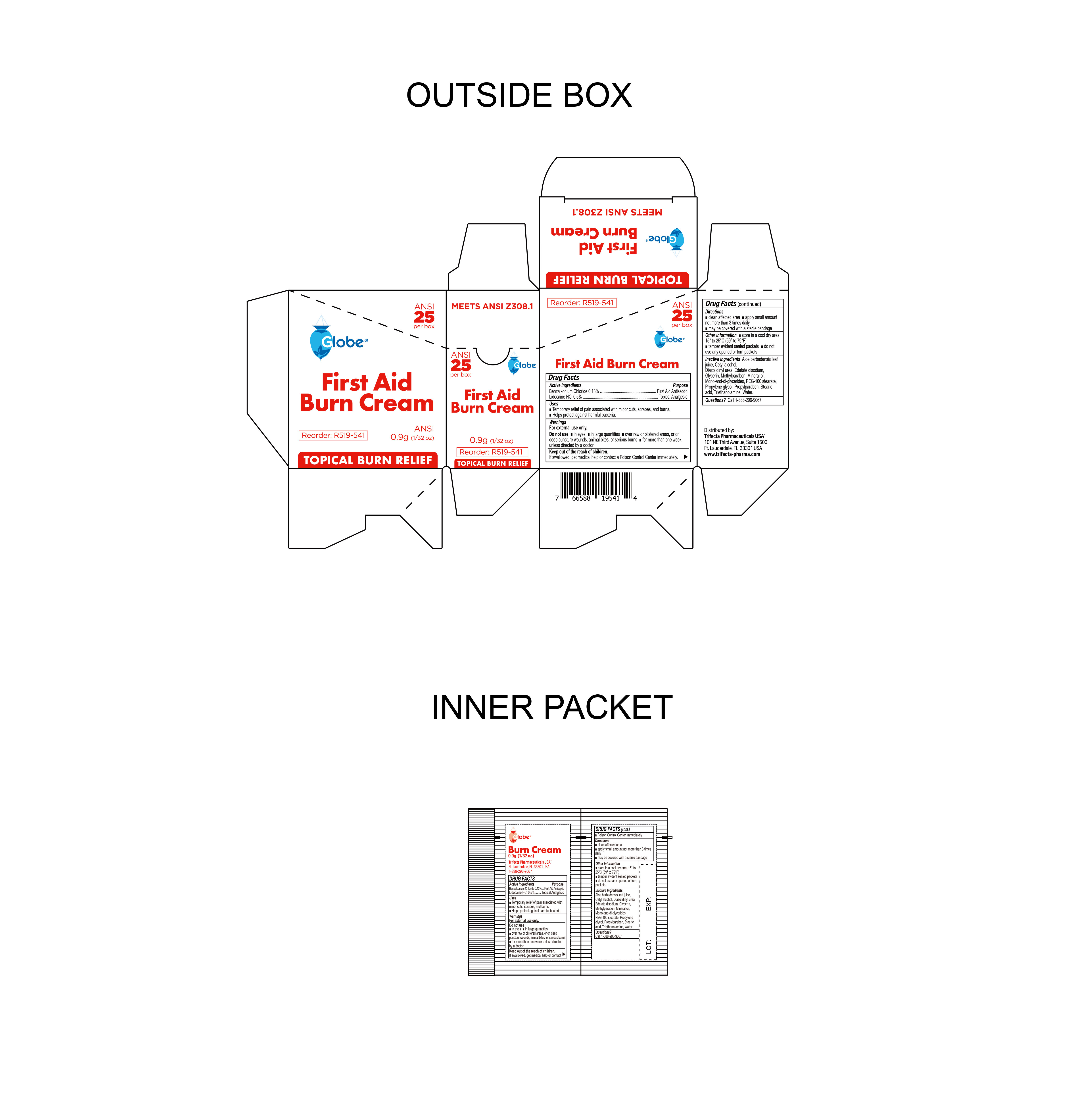
- Package Label
- Package Label
-
INGREDIENTS AND APPEARANCE
BURN CREAM
benzalkonium chloride, lidocaine hci creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-136 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.005 g in 1 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.0013 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) MINERAL OIL (UNII: T5L8T28FGP) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERIN (UNII: PDC6A3C0OX) TROLAMINE (UNII: 9O3K93S3TK) GLYCERYL MONO AND DIPALMITOSTEARATE (UNII: KC98RO82HJ) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) CETYL ALCOHOL (UNII: 936JST6JCN) PEG-100 STEARATE (UNII: YD01N1999R) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-136-10 10 in 1 CARTON 10/23/2023 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:69396-136-25 25 in 1 CARTON 10/23/2023 2 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/23/2023 Labeler - Trifecta Pharmaceuticals USA LLC (079424163)