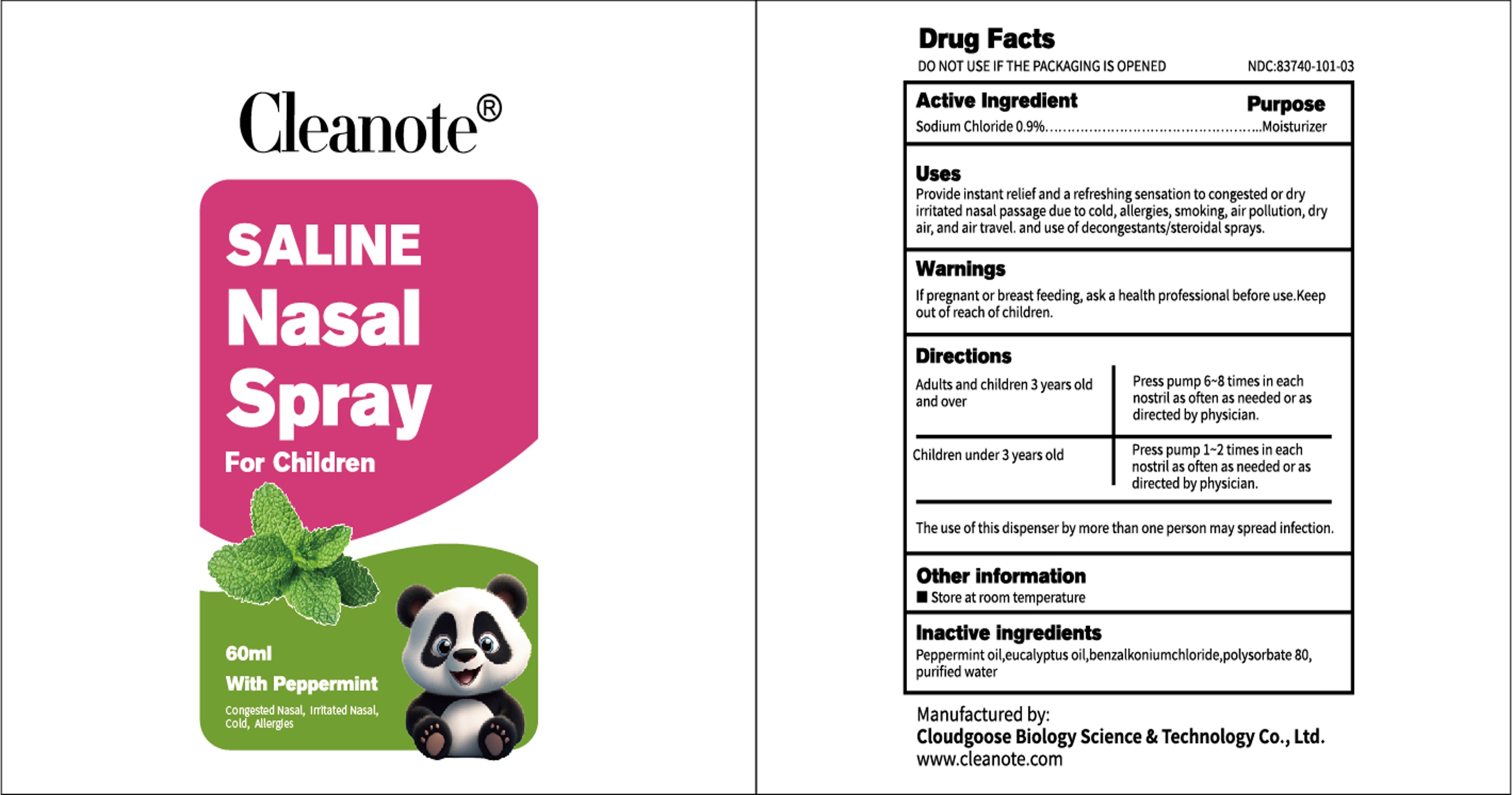
Label: CLEANOTE SALINE NASAL SPRAYS- sodium chloride 0.9% aerosol, spray
- NDC Code(s): 83740-101-01, 83740-101-02, 83740-101-03
- Packager: Cloudgoose Biology Science & Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEANOTE SALINE NASAL SPRAYS
sodium chloride 0.9% aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83740-101 Route of Administration INTRASINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698, SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 9 mg in 100 mL Inactive Ingredients Ingredient Name Strength PEPPERMINT OIL (UNII: AV092KU4JH) EUCALYPTUS OIL (UNII: 2R04ONI662) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83740-101-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2023 2 NDC:83740-101-02 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2023 3 NDC:83740-101-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/23/2023 Labeler - Cloudgoose Biology Science & Technology Co., Ltd. (420735544) Registrant - Cloudgoose Biology Science & Technology Co., Ltd. (420735544) Establishment Name Address ID/FEI Business Operations Cloudgoose Biology Science & Technology Co., Ltd. 420735544 manufacture(83740-101)